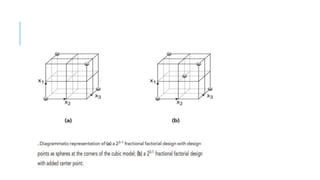
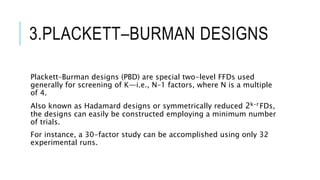
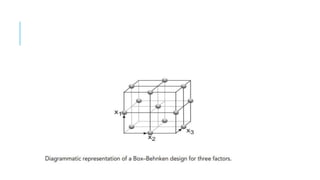
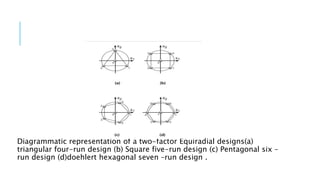
This document provides an overview of optimization techniques used in pharmaceutical formulation and processing. It discusses key concepts like the definition of optimization, advantages of systematic optimization approaches, and common optimization parameters. It also describes important terms in optimization like factors, effects, interactions, coding, and confounding. Different experimental design approaches are explained, including factorial designs, fractional factorial designs, Plackett-Burman designs, central composite designs, Box-Behnken designs, and mixture designs. The document concludes by noting the seven main steps involved in the optimization methodology for drug delivery systems.