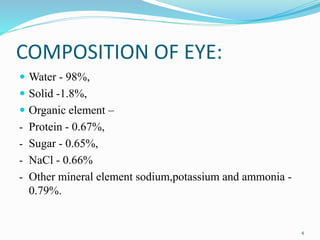
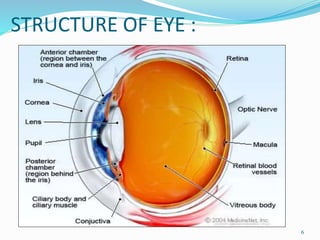
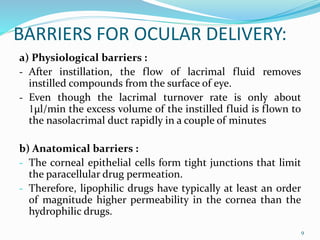
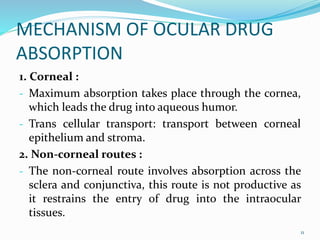
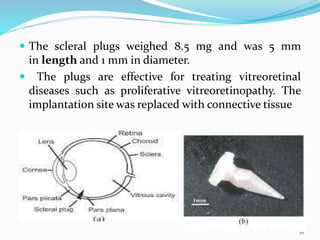
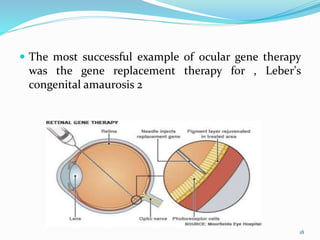
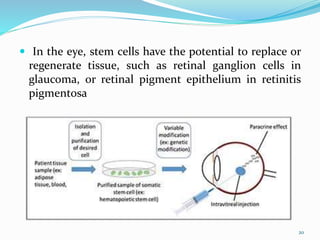
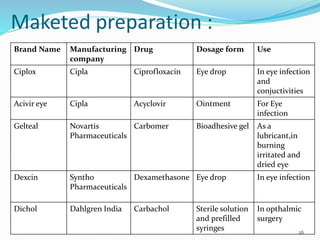
The document discusses ocular drug delivery systems. It begins with an introduction to ocular drug delivery and the challenges associated with it. It then describes the anatomy and structures of the eye. The main routes of ocular drug delivery are topical, subconjunctival, and intravitreal administration. Barriers to delivery include physiological barriers like tear turnover and anatomical barriers like tight corneal cell junctions. Various ocular drug delivery systems are outlined including conventional, vesicular, controlled release, and particulate systems. Emerging advanced systems like scleral plugs, gene therapy, and stem cell therapy are also summarized. Evaluation methods for different ocular formulations and some examples of marketed ophthalmic products are provided.