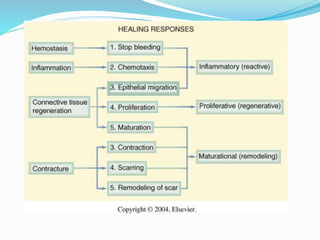
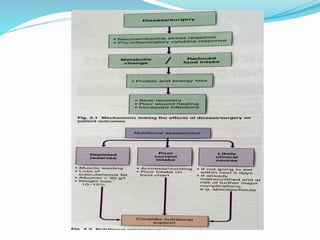
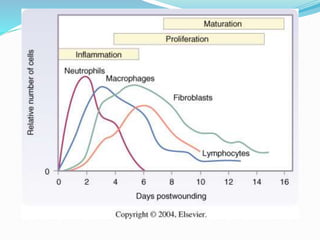
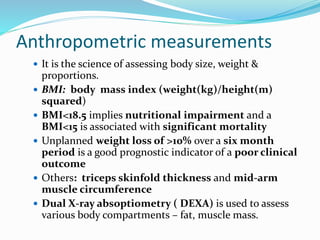
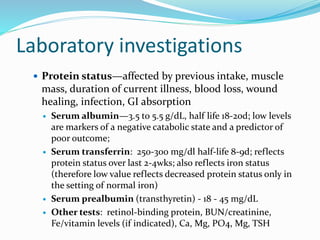
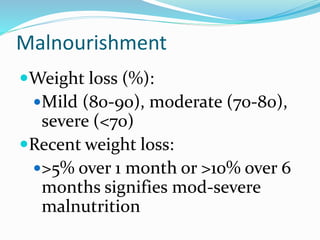
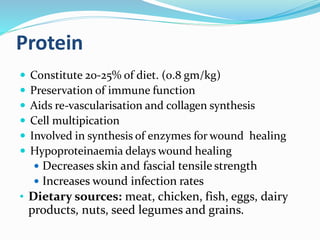
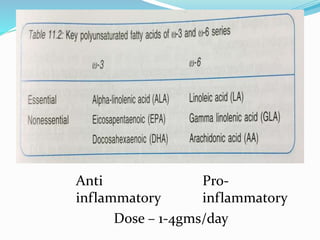
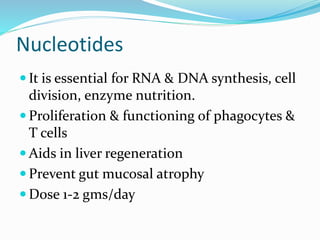
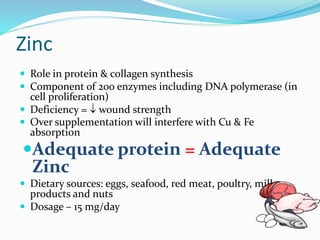
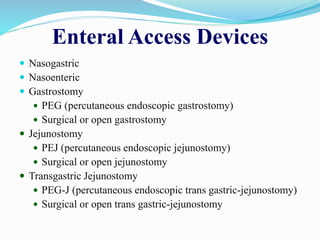
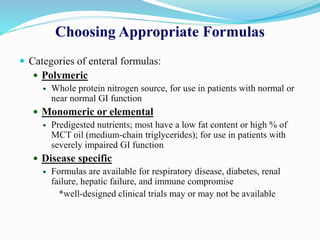
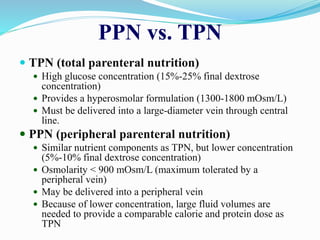
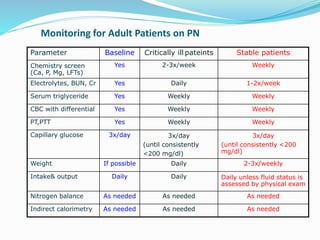
This document discusses the role of nutrition in wound healing. Nutrition plays a vital role throughout all stages of wound healing, including the inflammatory, proliferative, and remodeling phases. Adequate intake of nutrients is necessary for processes like tissue growth and repair during healing. Malnutrition can negatively impact wound healing by impairing the immune system and decreasing wound strength. Several key nutrients are discussed in detail that are important for wound healing, including proteins, vitamins A and C, zinc, and amino acids like glutamine and arginine. The document also covers nutrition support and enteral access devices when oral intake is not sufficient.