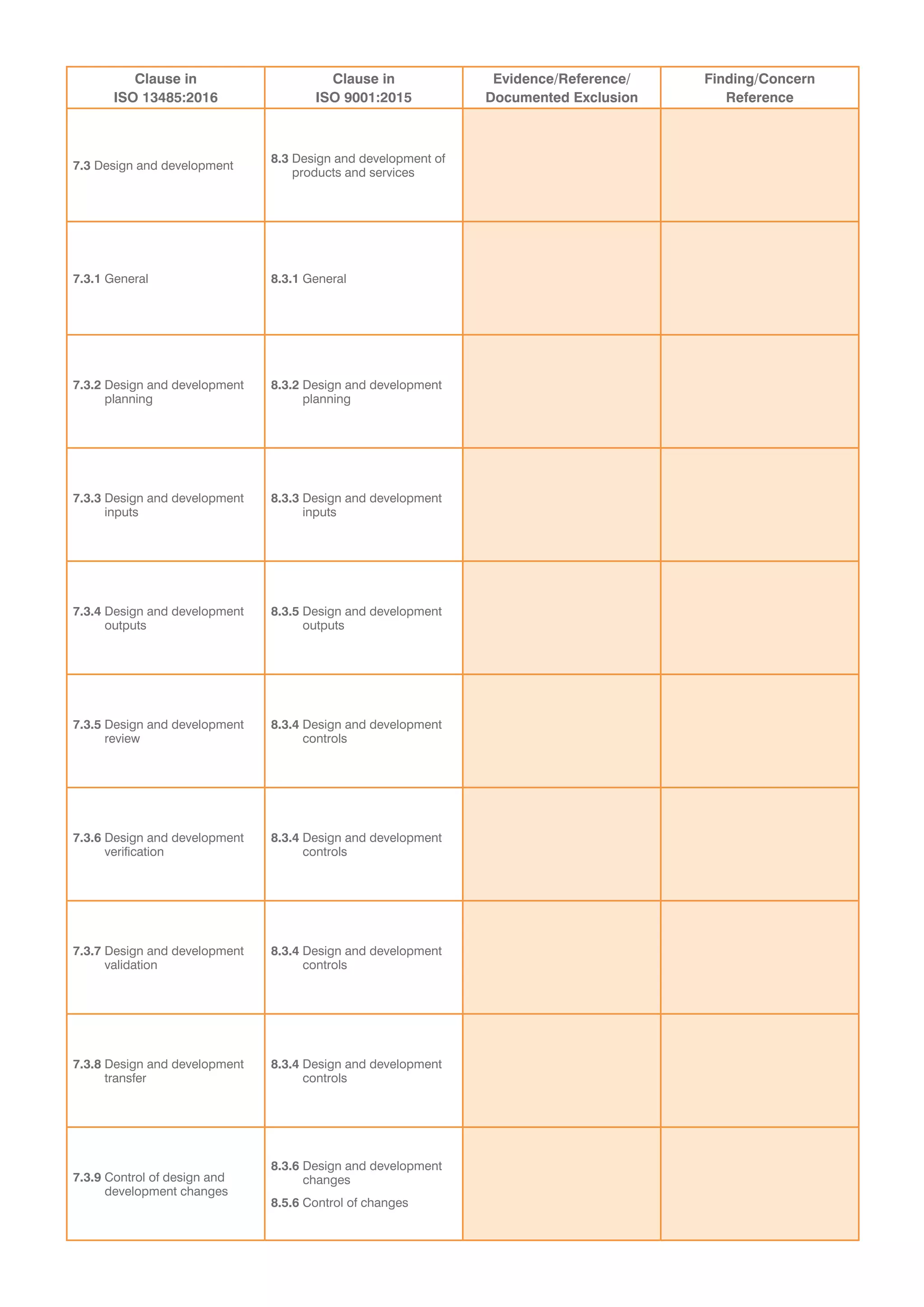
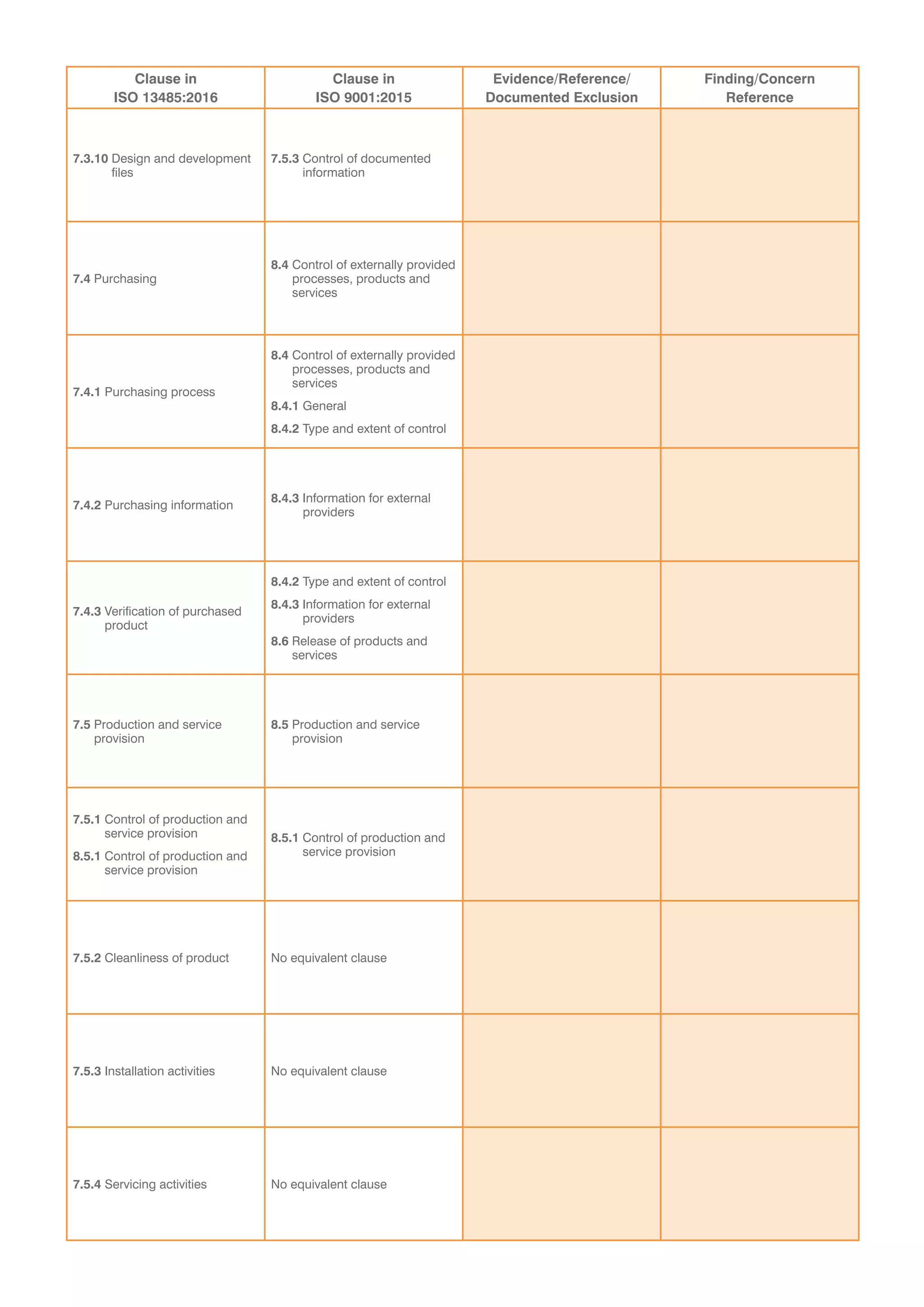
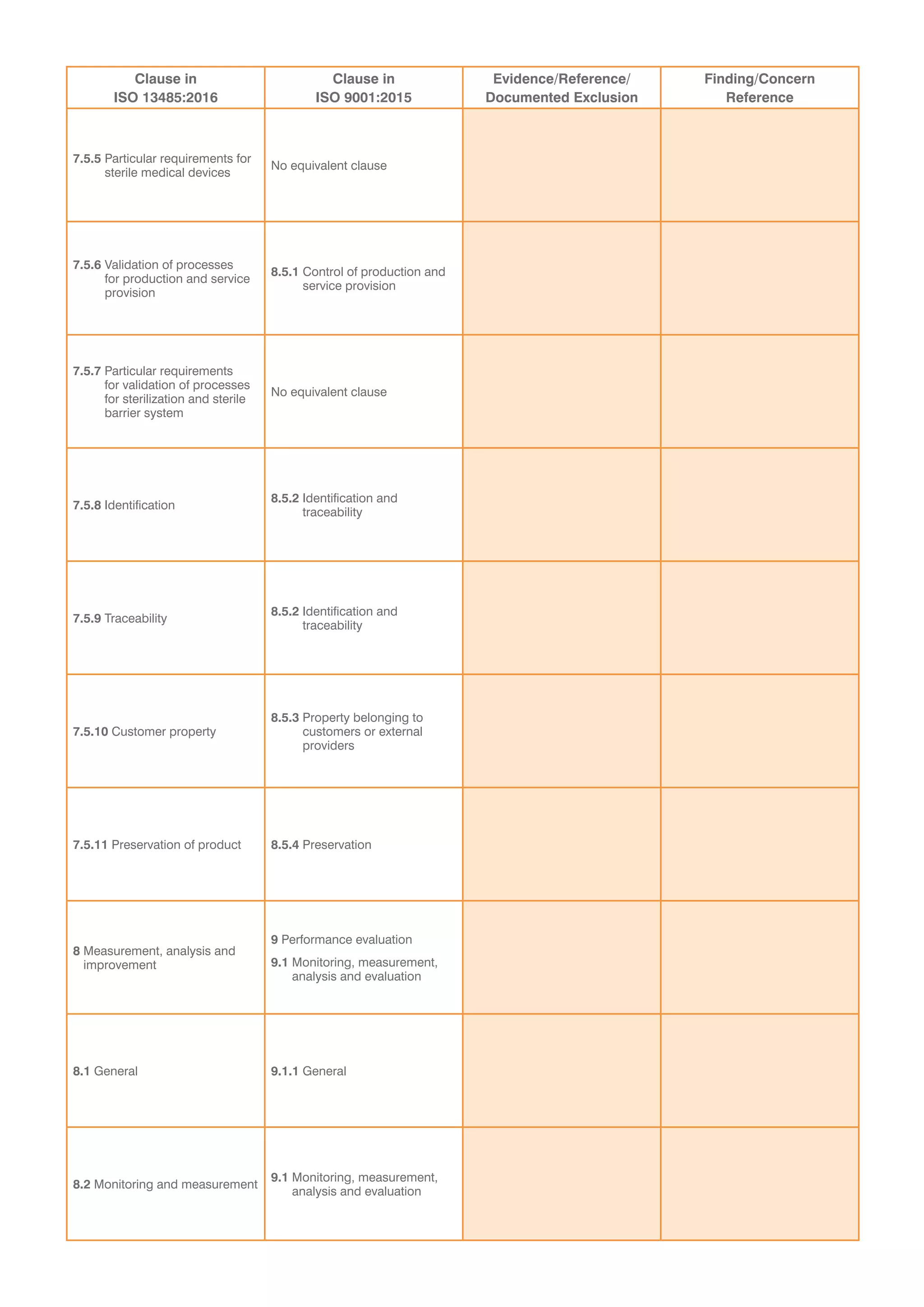
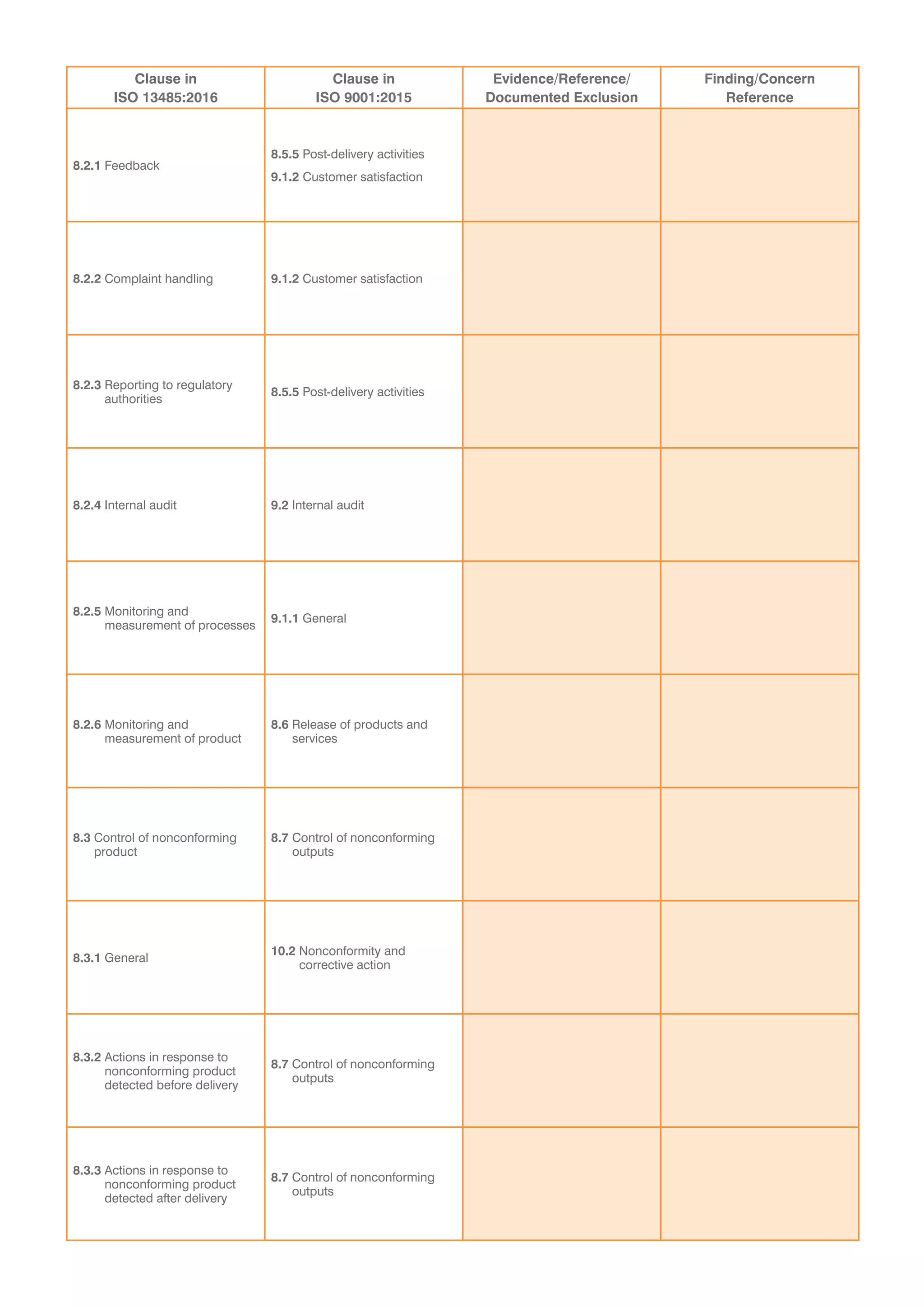
This document provides a checklist for organizations transitioning from ISO 9001:2008 to ISO 13485:2016 and ISO 9001:2015. It contains tables to validate that an organization's integrated quality management system addresses new concepts in both standards. The tables list clauses from each standard and require evidence that requirements have been met without exceptions. It concludes that a recommendation for certification is dependent on submitting a corrective action plan to address any findings from the audit, or that a special on-site visit may be required if registration cannot be immediately recommended.