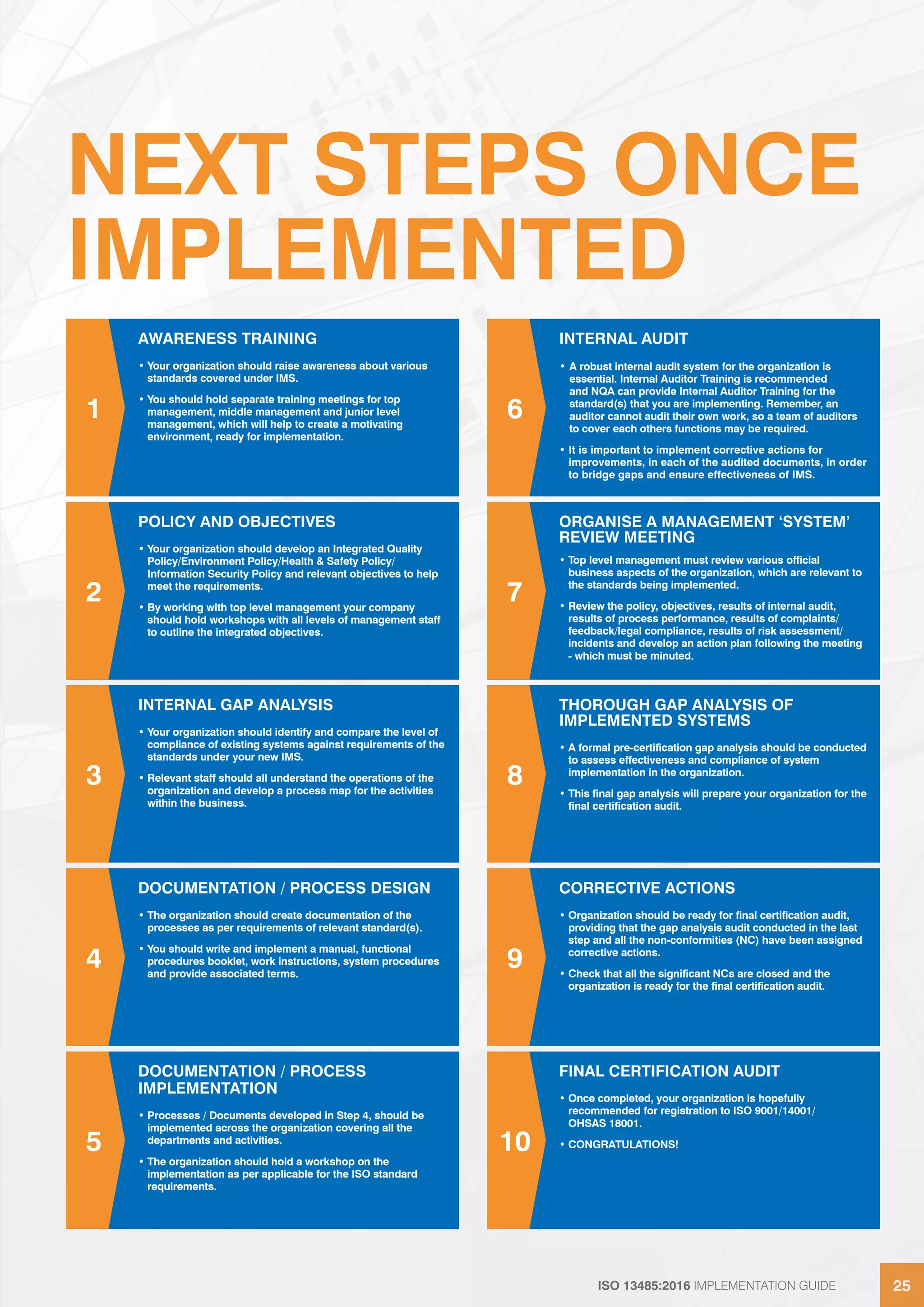
The ISO 13485:2016 implementation guide outlines requirements for quality management systems in medical device manufacturing, emphasizing regulatory compliance and customer needs. It highlights the importance of effective leadership, risk-based thinking, and process management to enhance quality and efficiency. The standard facilitates global alignment of regulatory requirements and supports organizations in consistently delivering safe and effective medical devices.