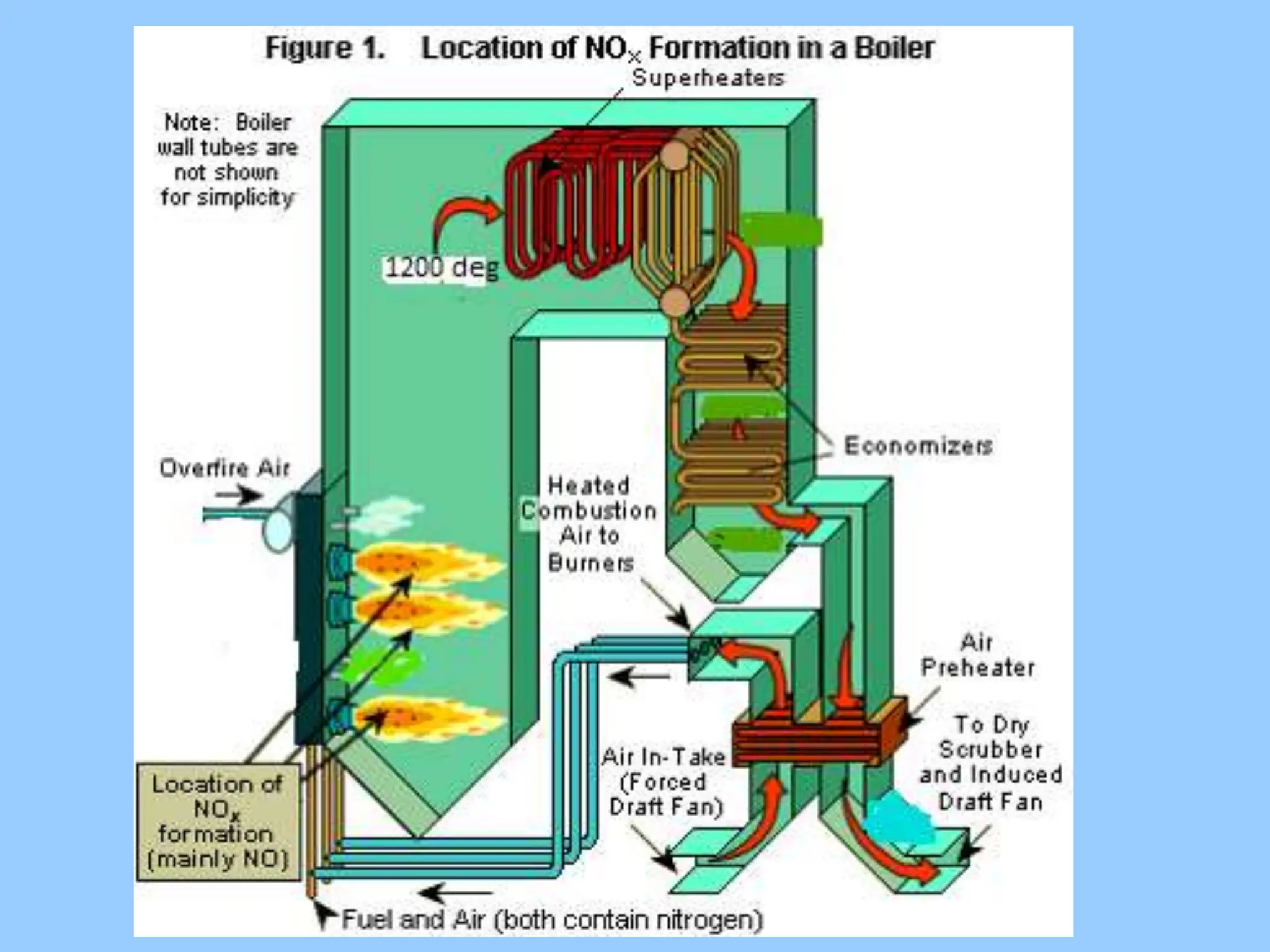
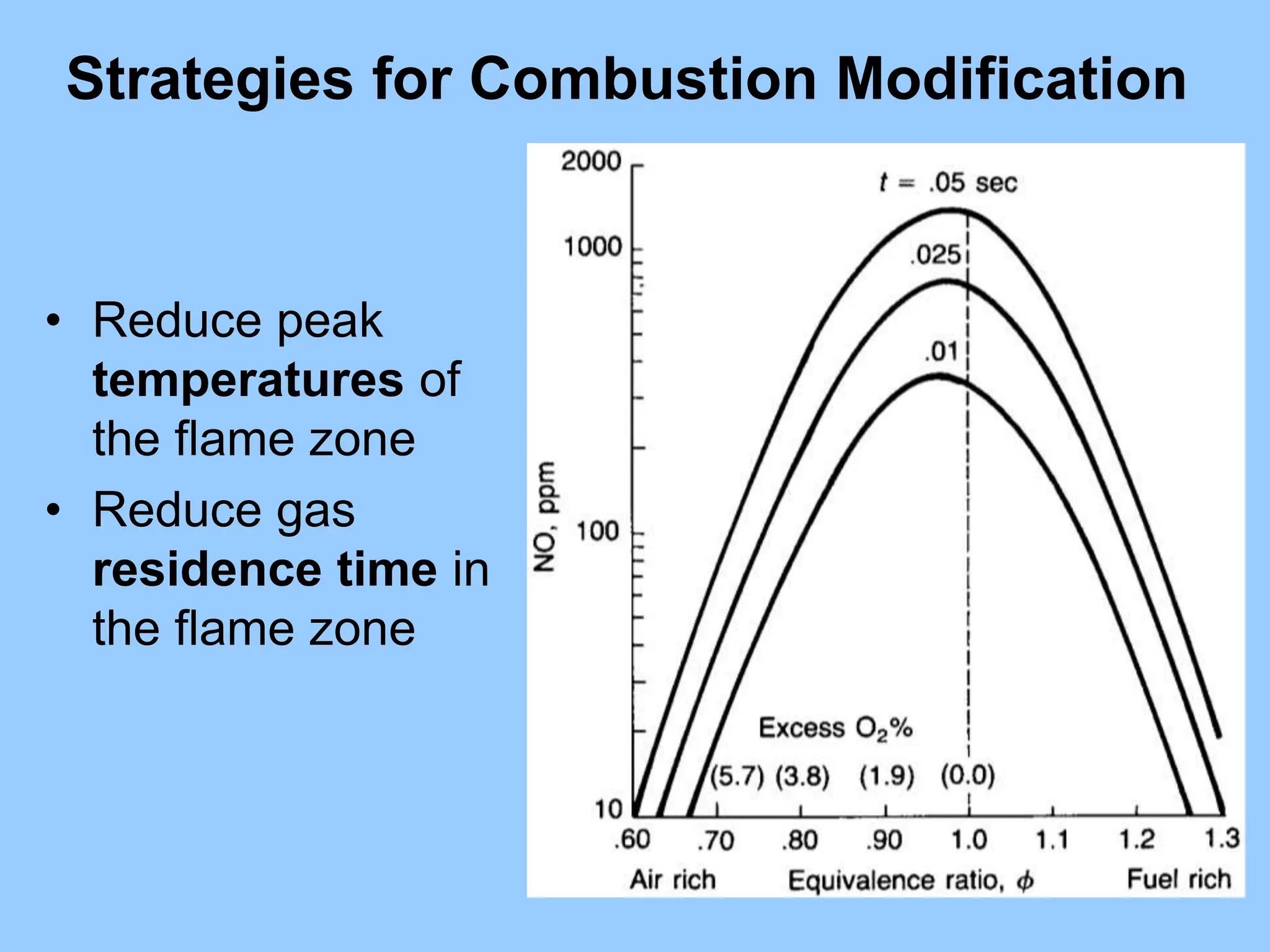
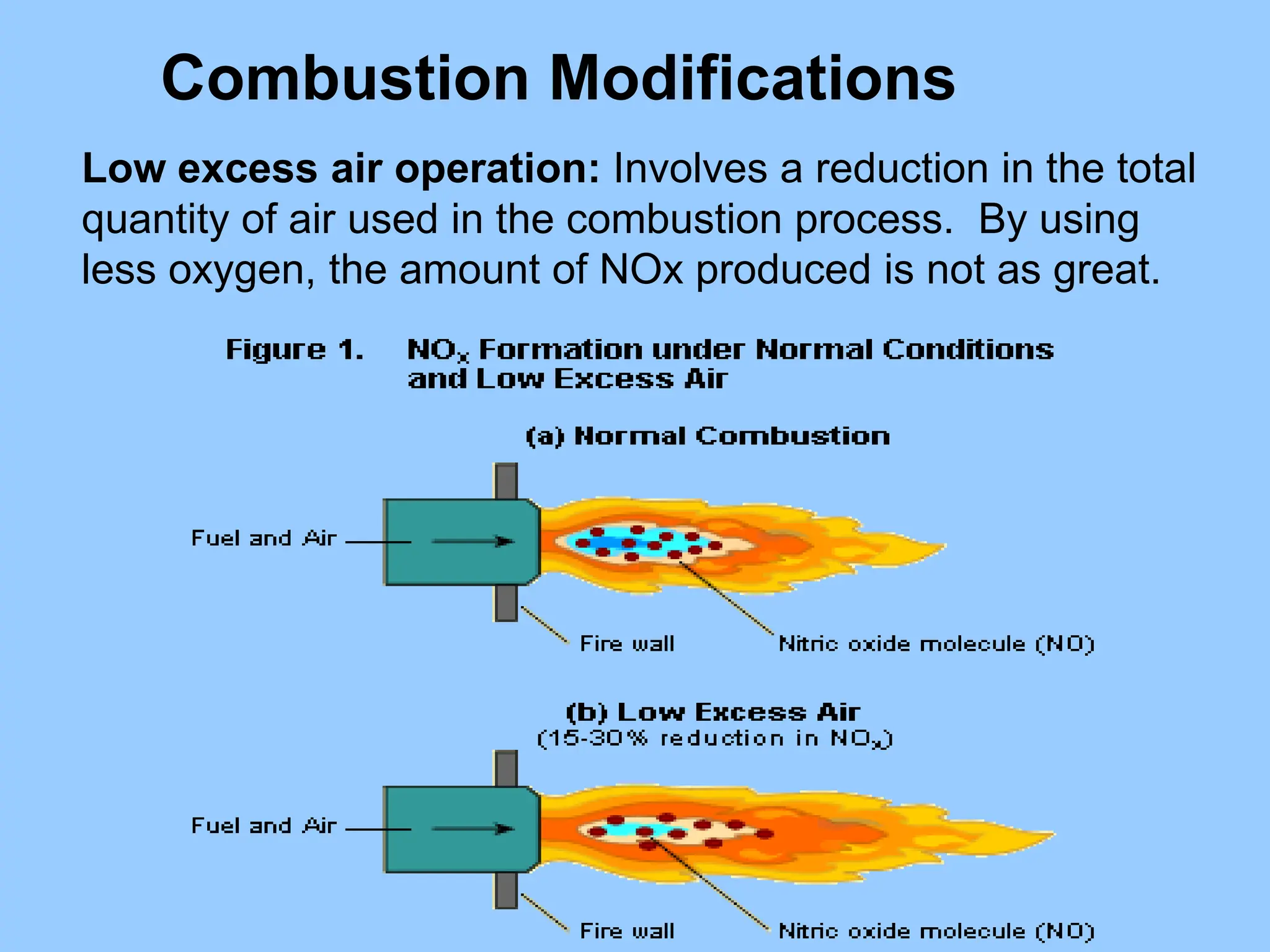
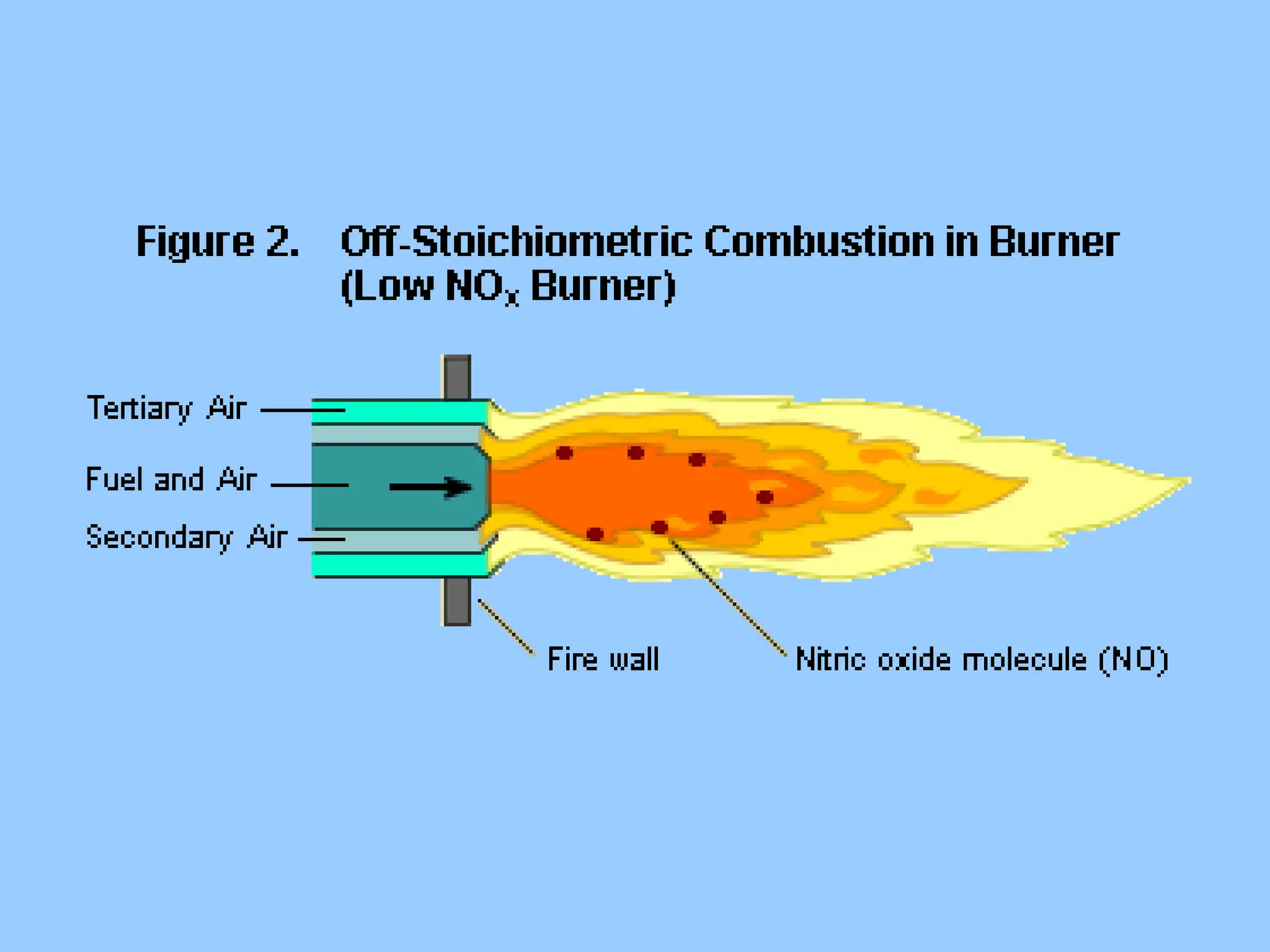
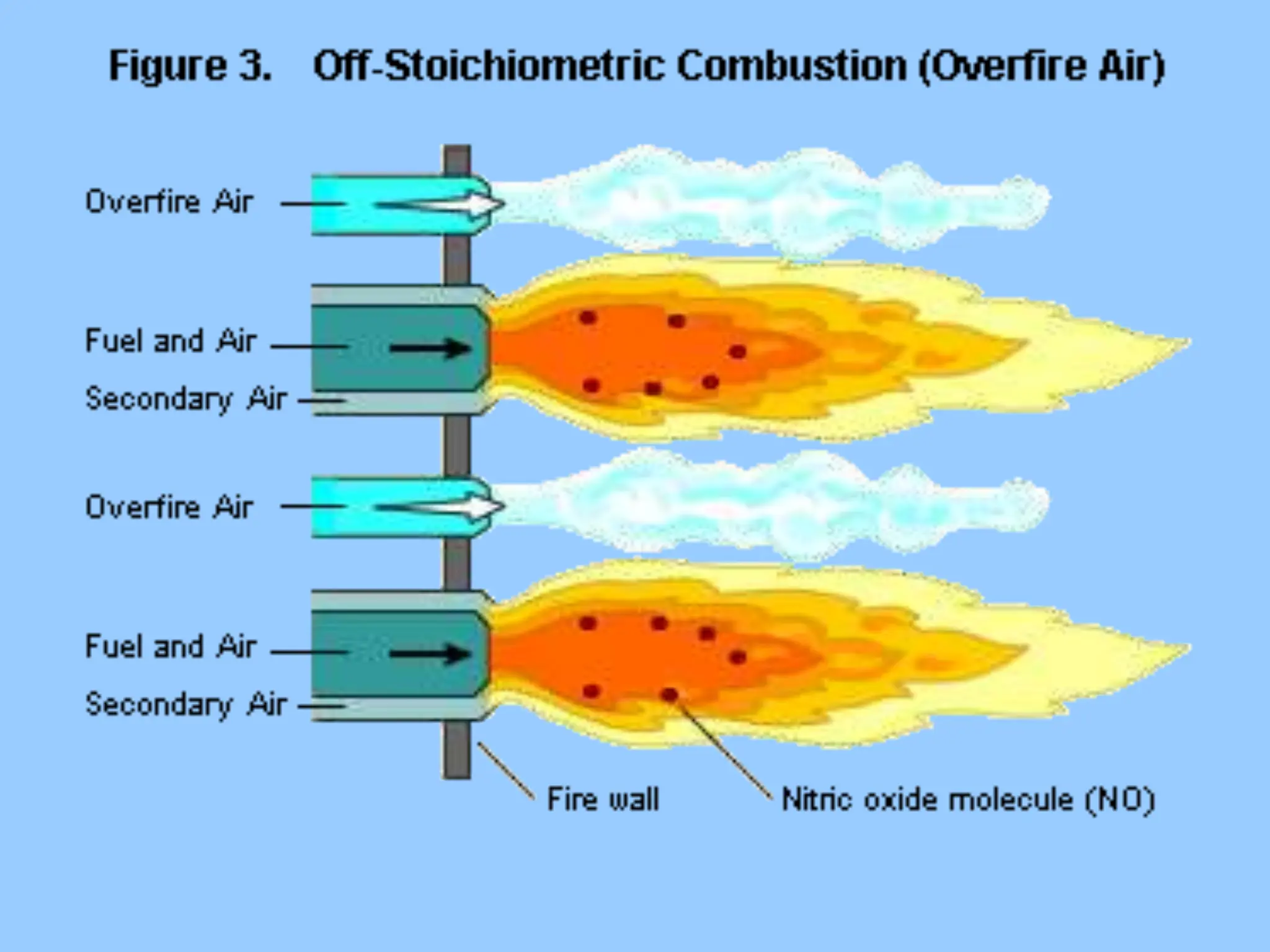
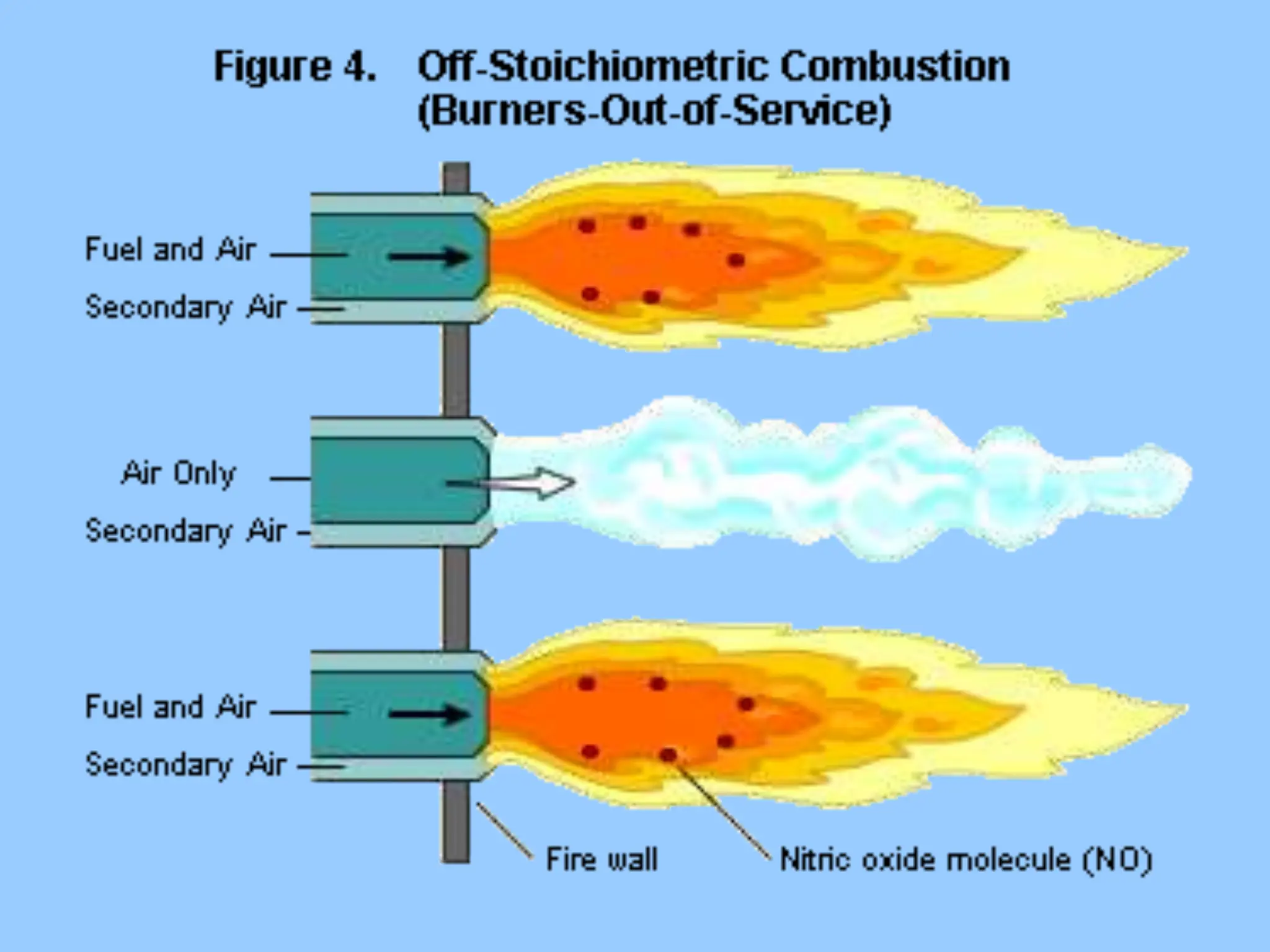
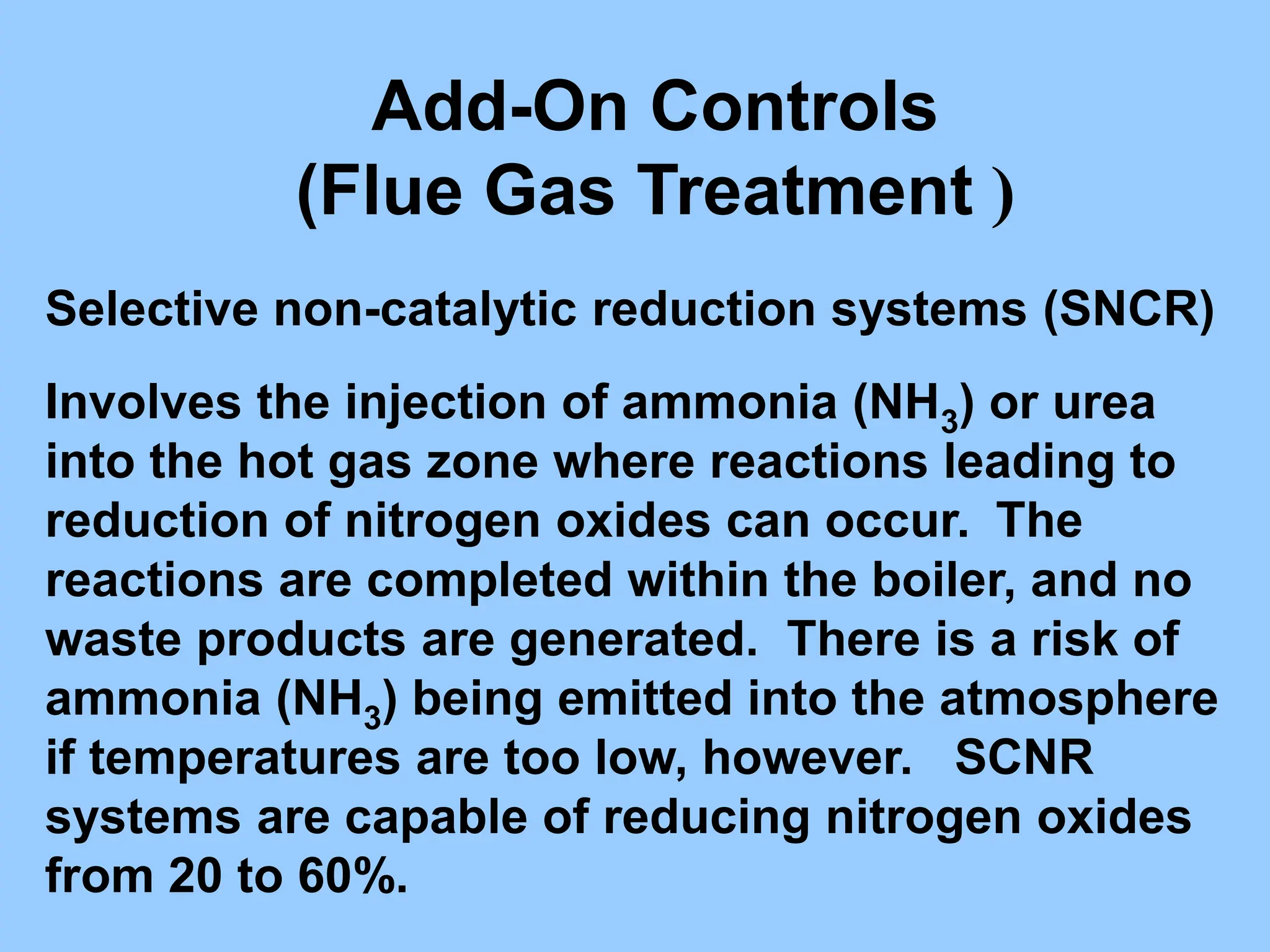
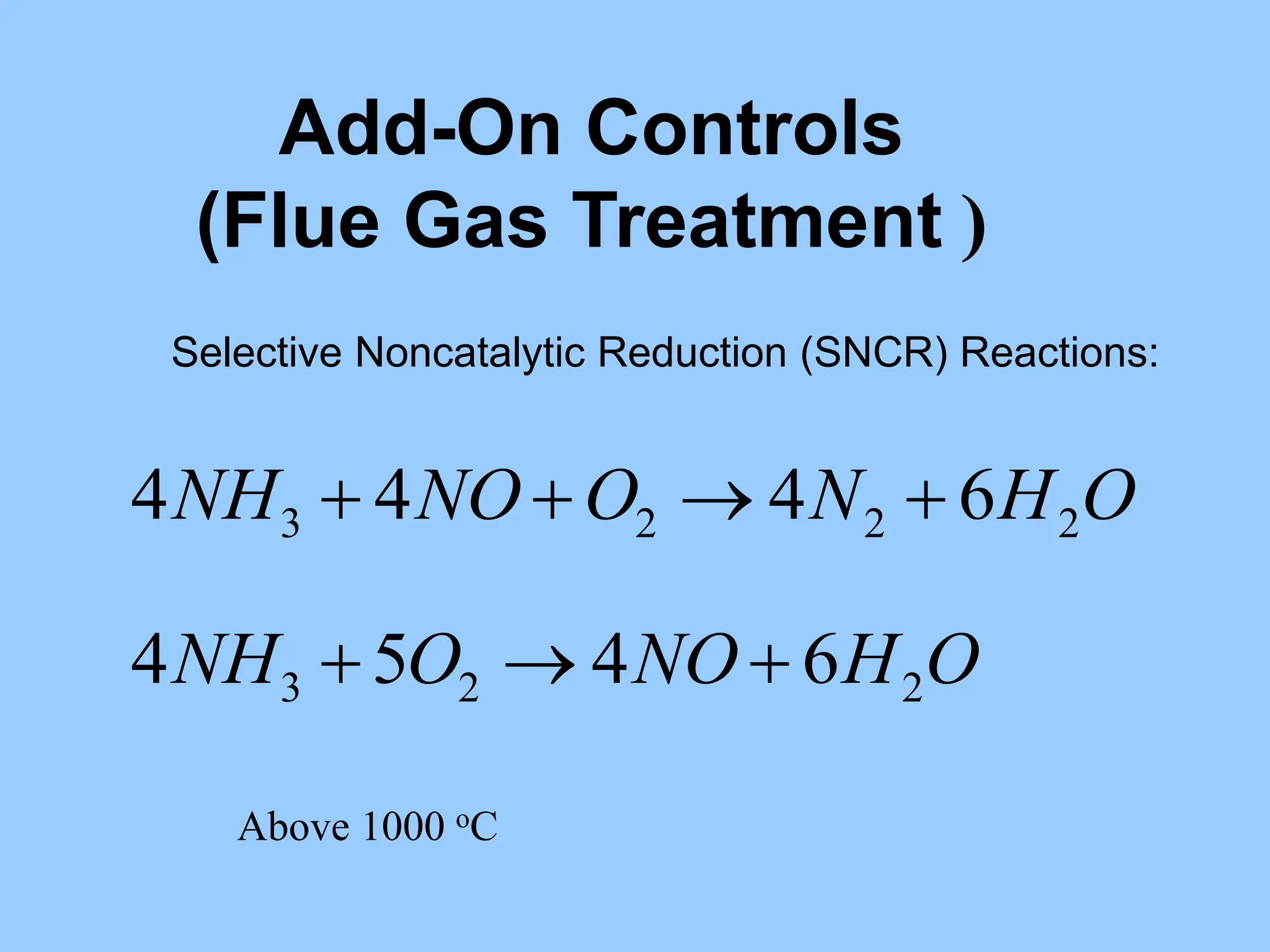
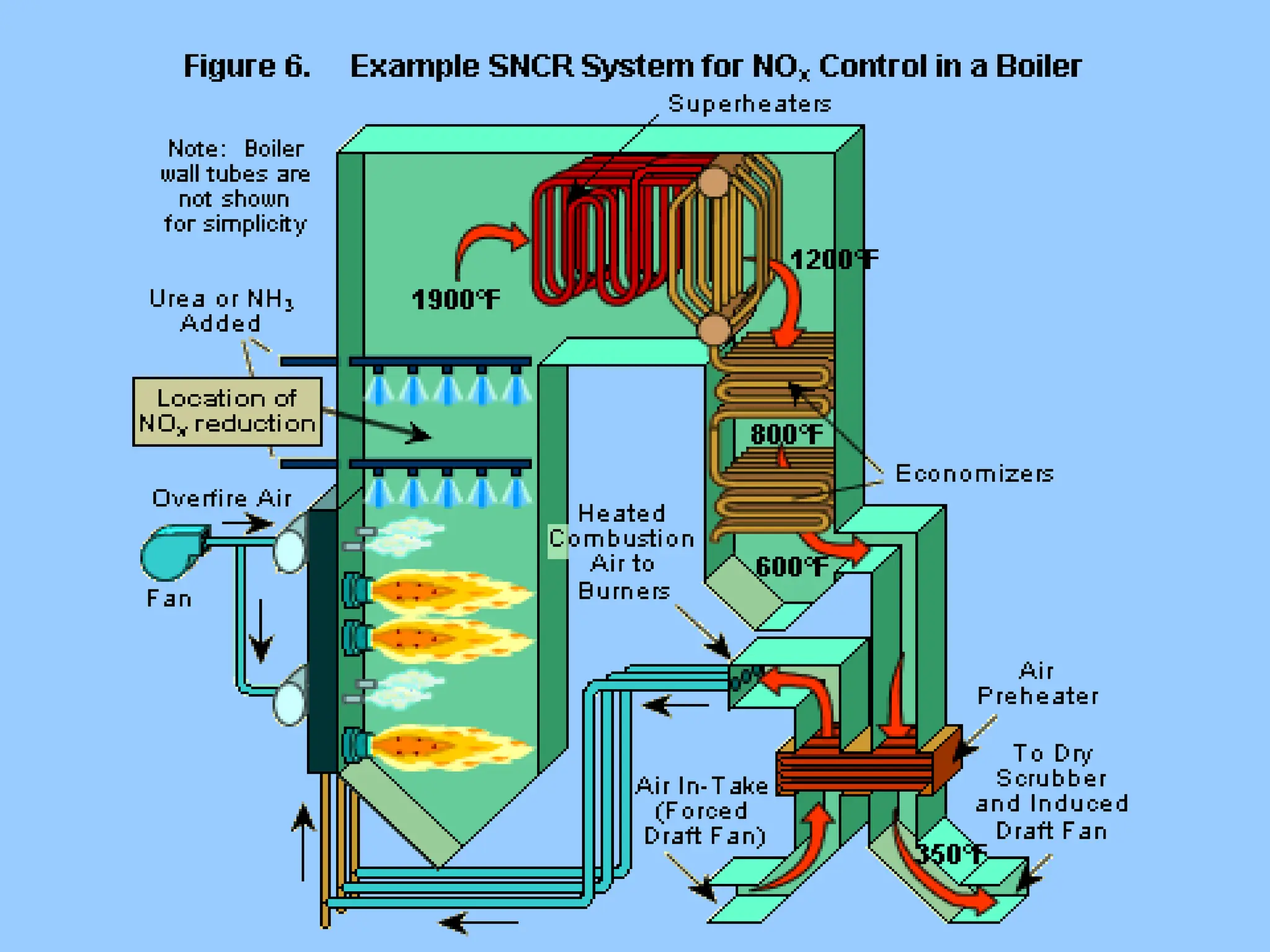
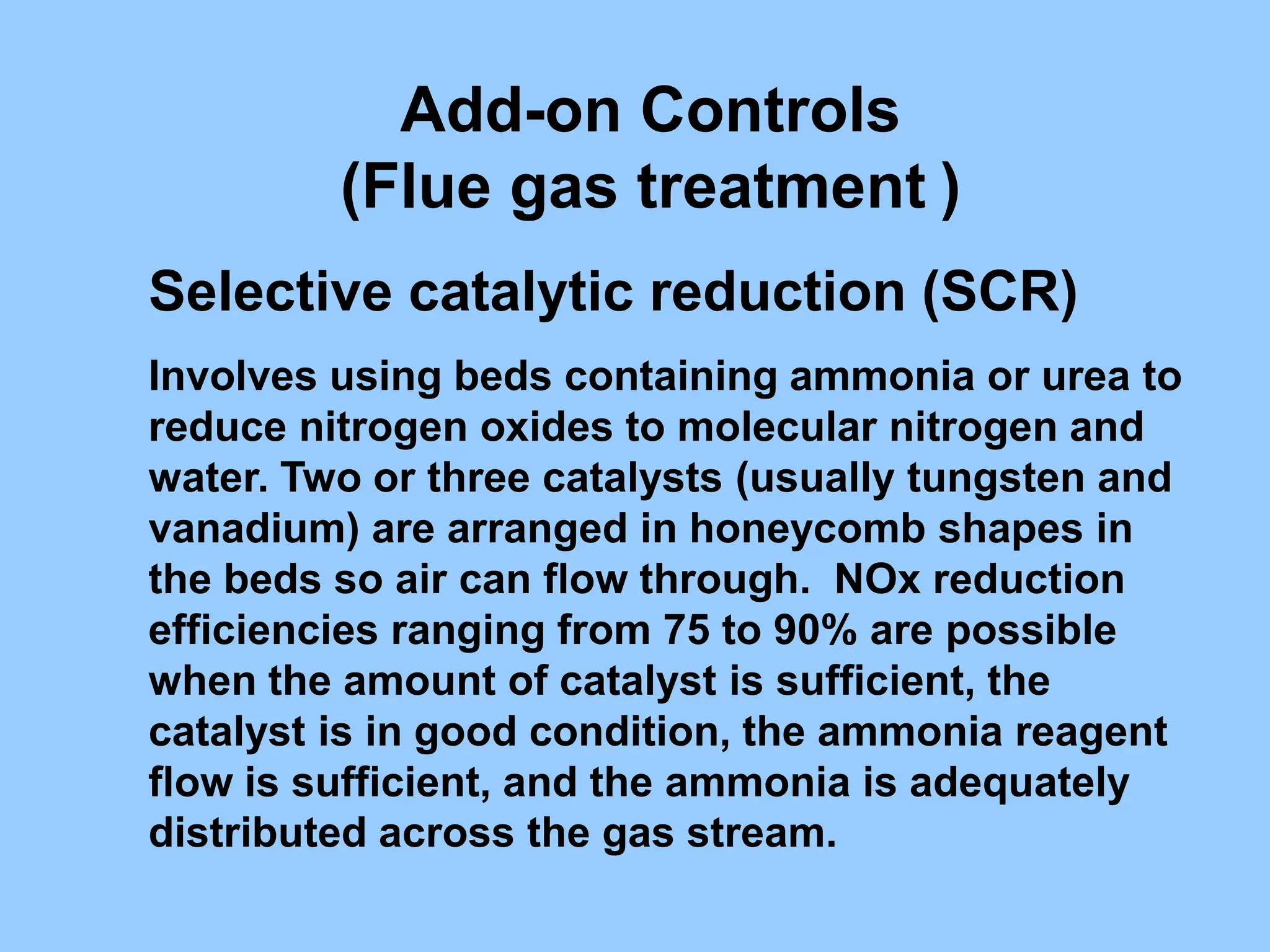
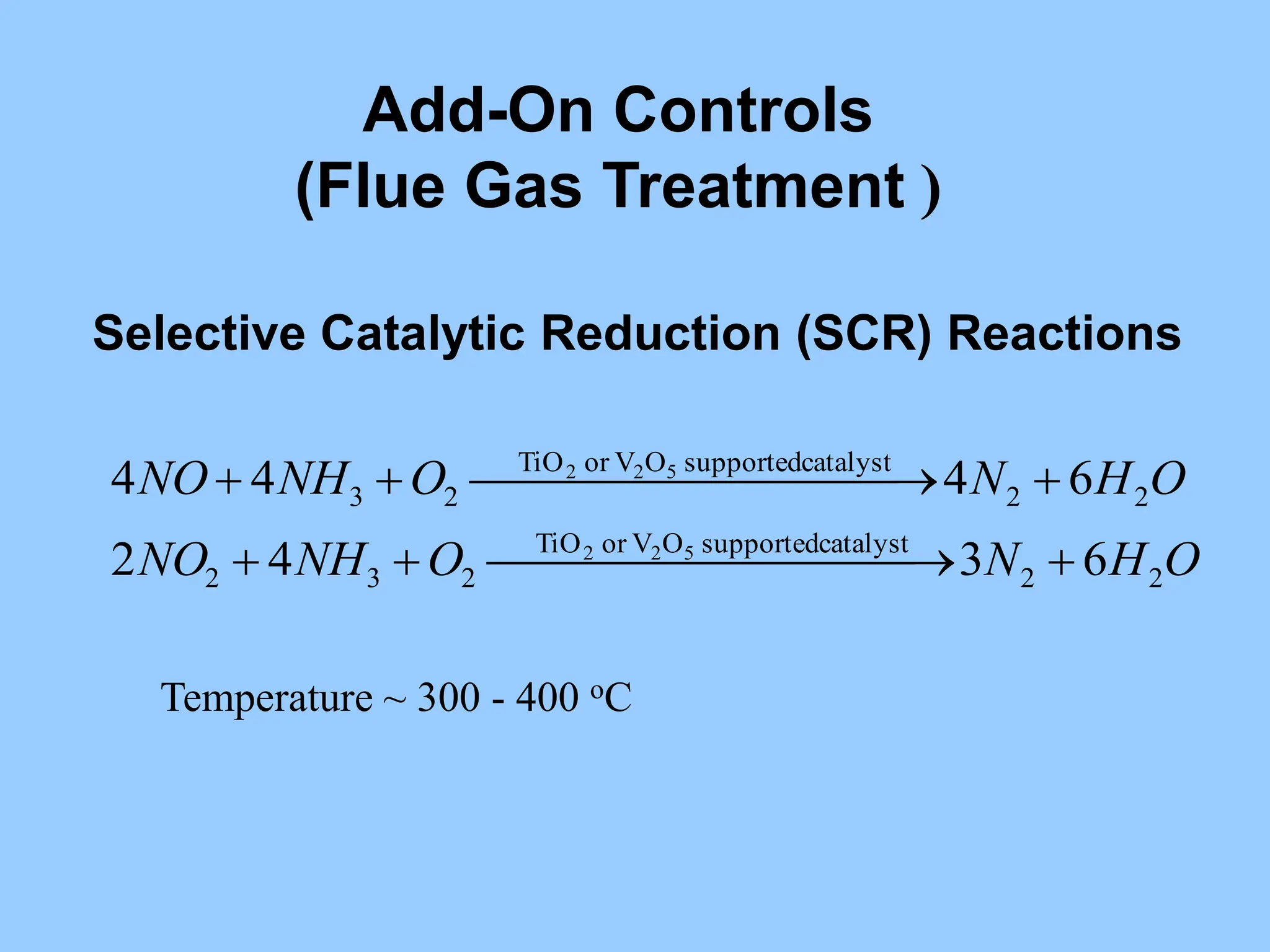
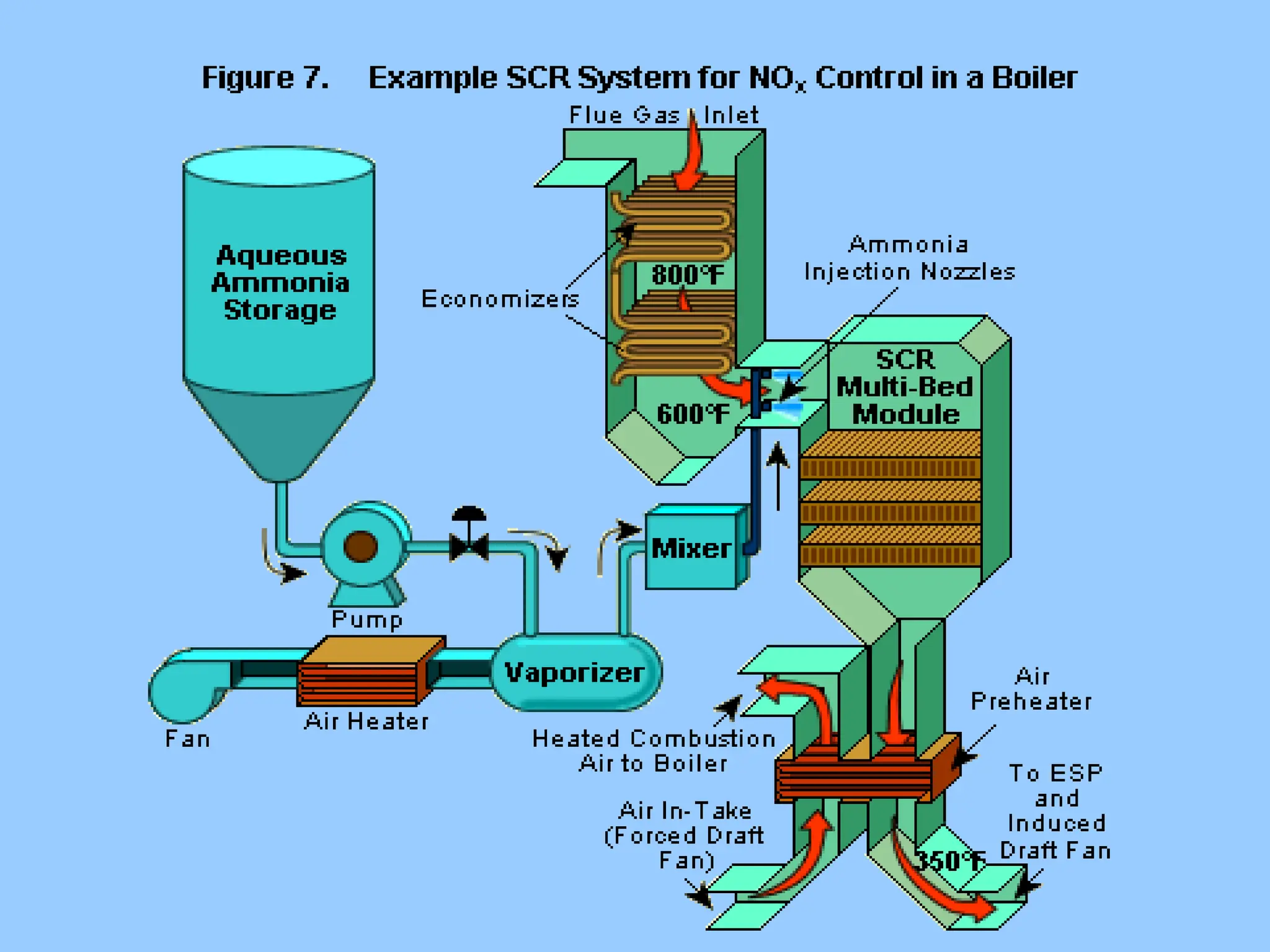
The document discusses the characteristics, formation, and control of nitrogen oxides (NOx), particularly nitric oxide (NO) and nitrogen dioxide (NO2). NOx is primarily formed during high-temperature combustion processes and has significant environmental impacts, prompting the development of various control technologies such as combustion modifications and flue gas treatment methods like selective non-catalytic reduction (SNCR) and selective catalytic reduction (SCR). These technologies aim to reduce NOx emissions effectively through various chemical reactions and operational adjustments.