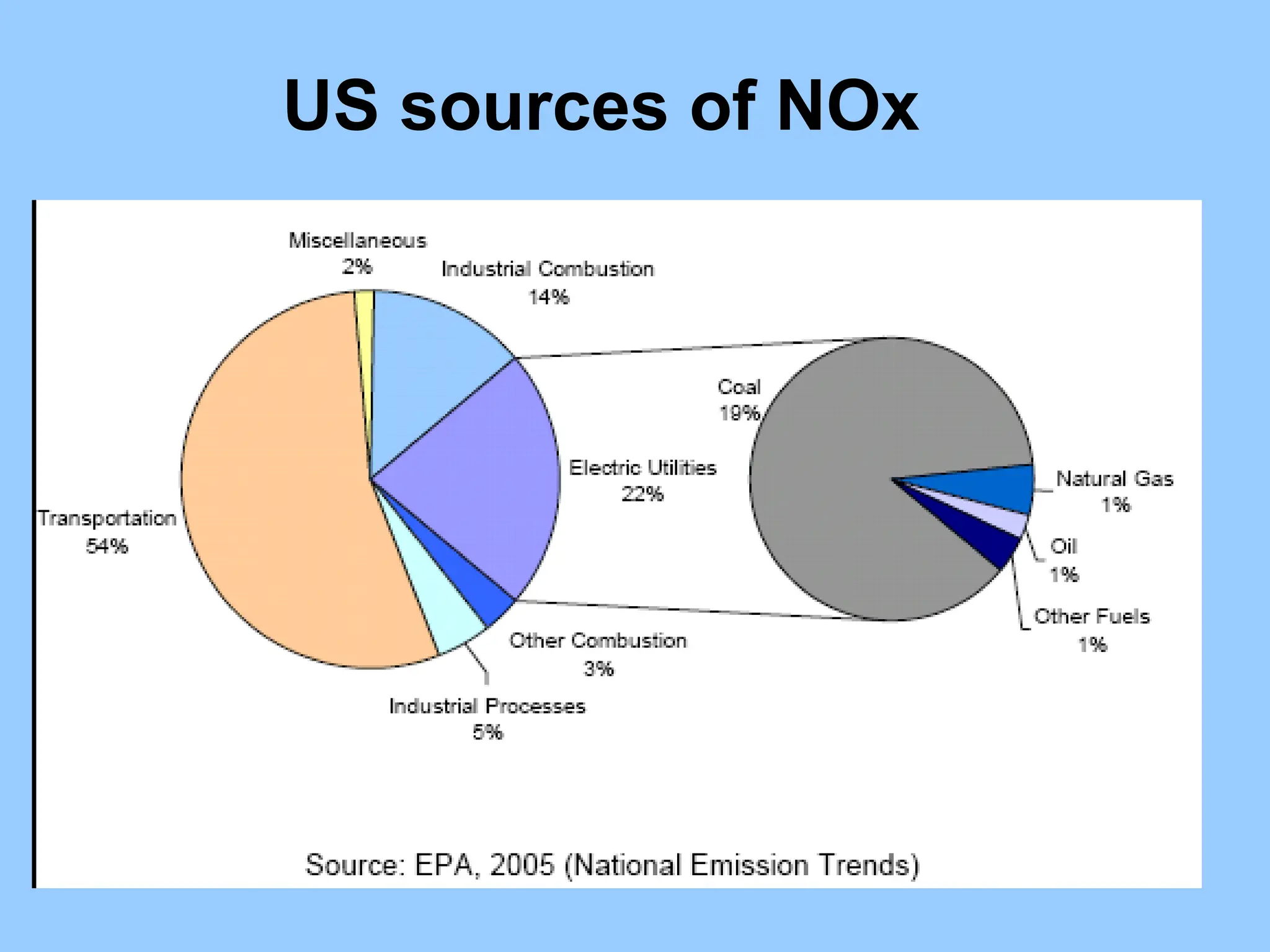
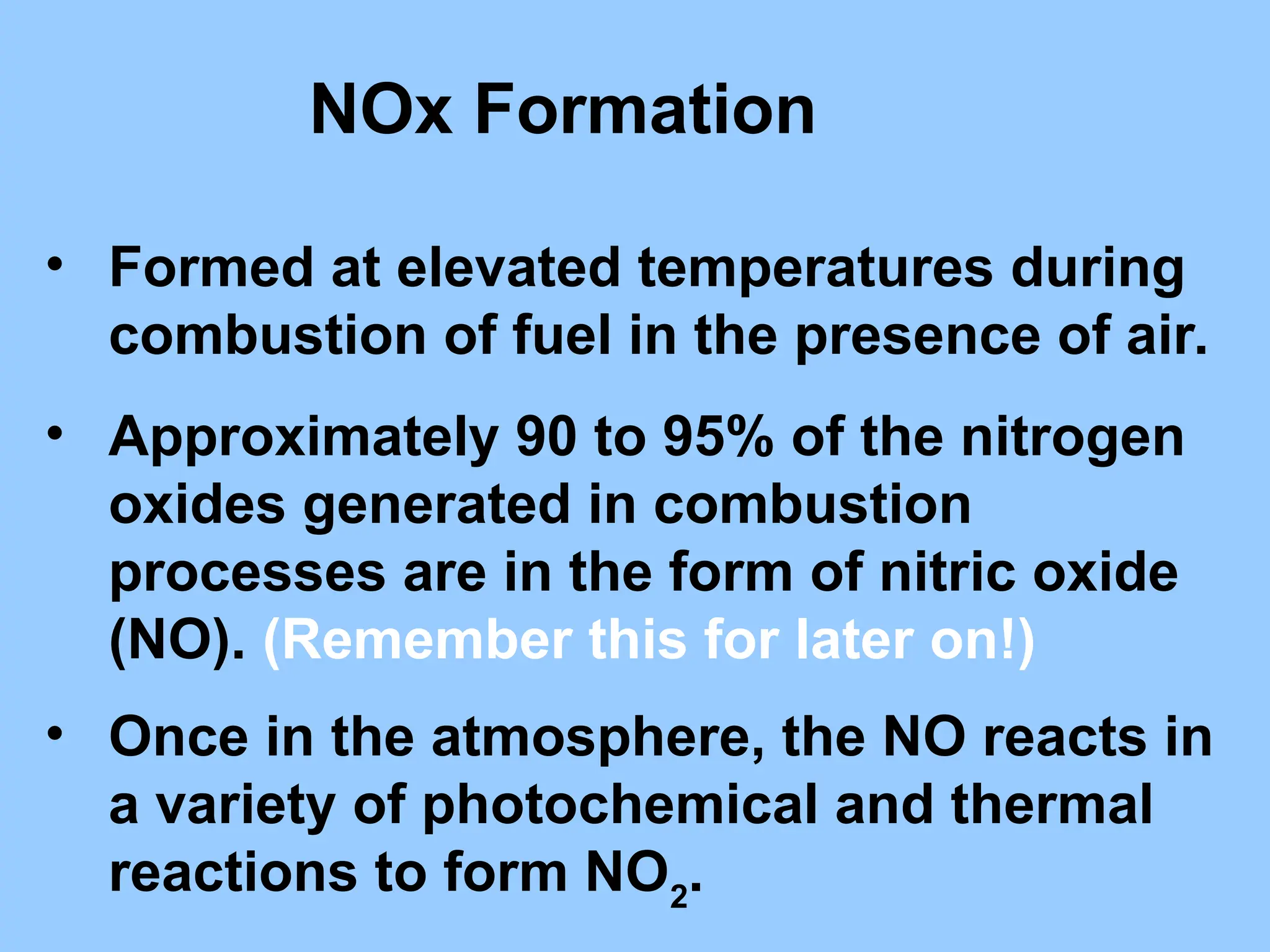
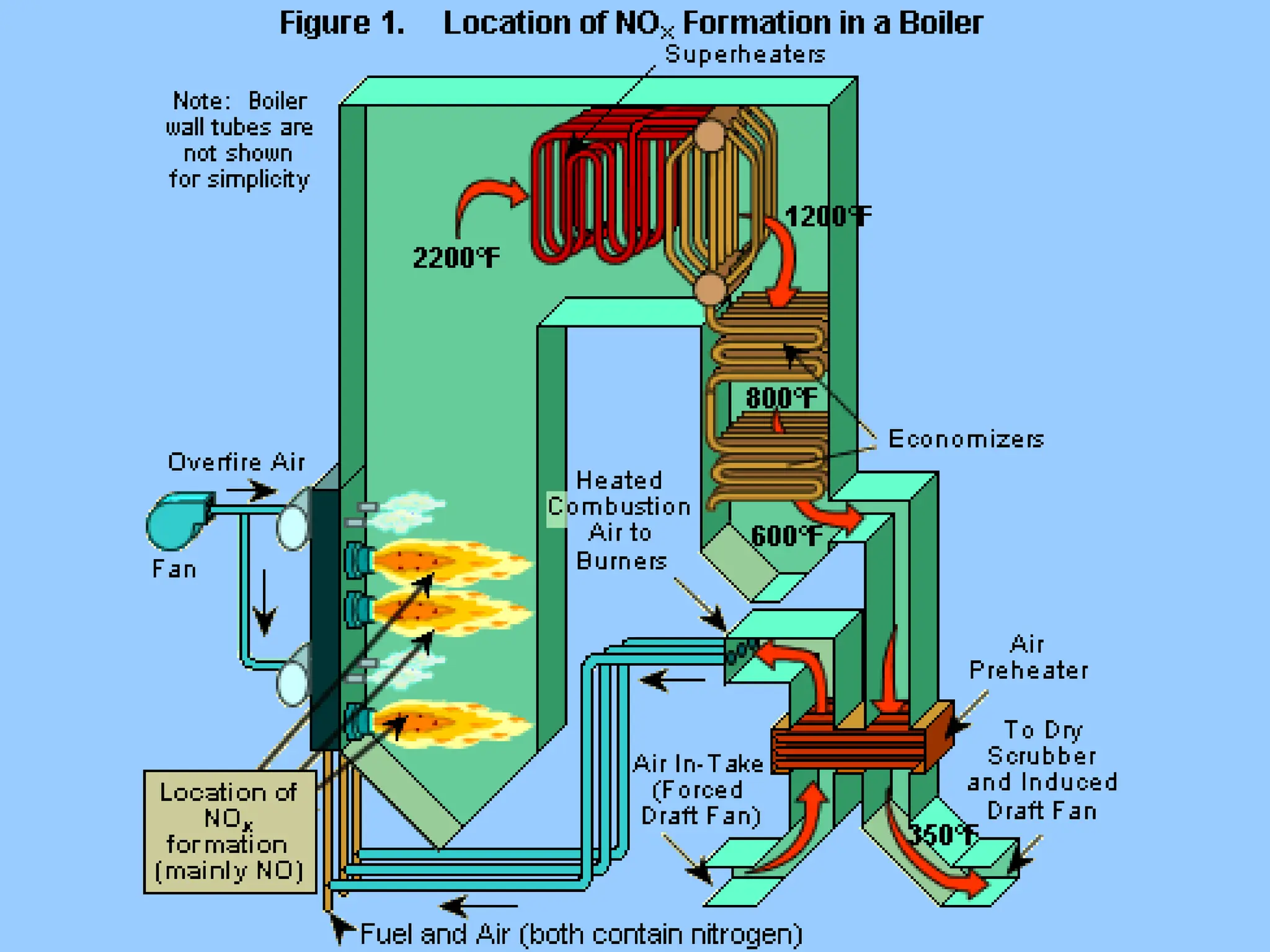
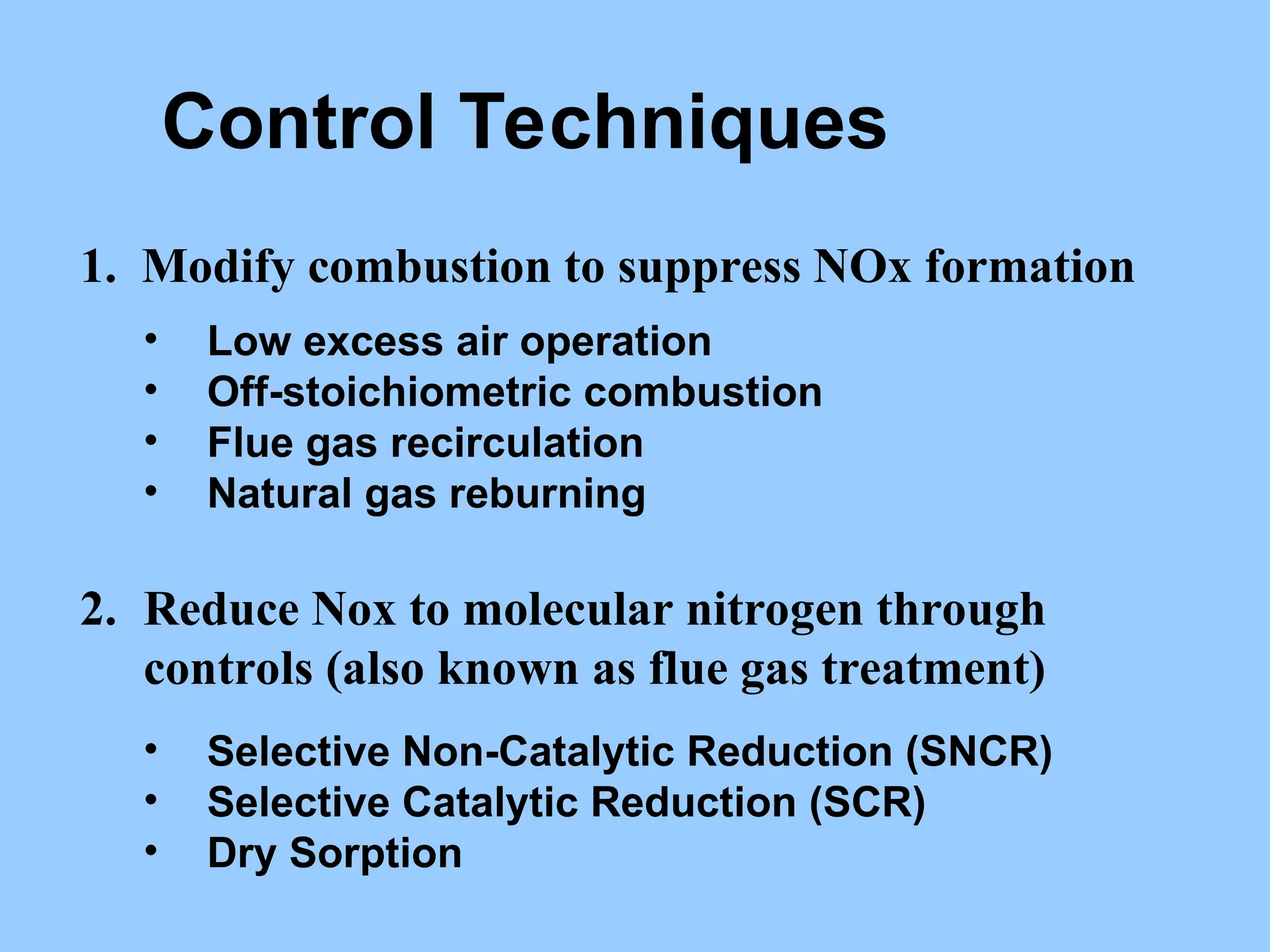
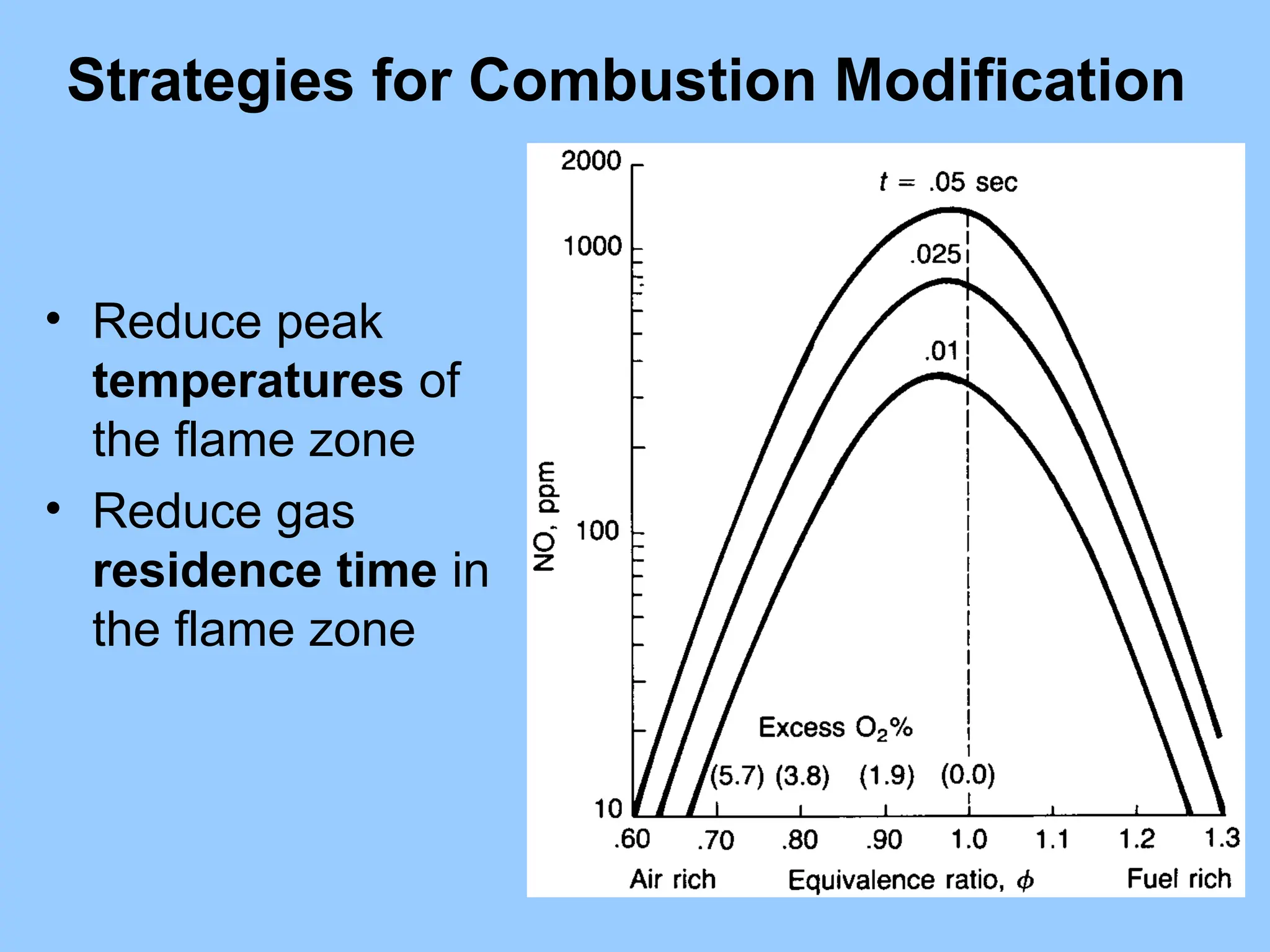
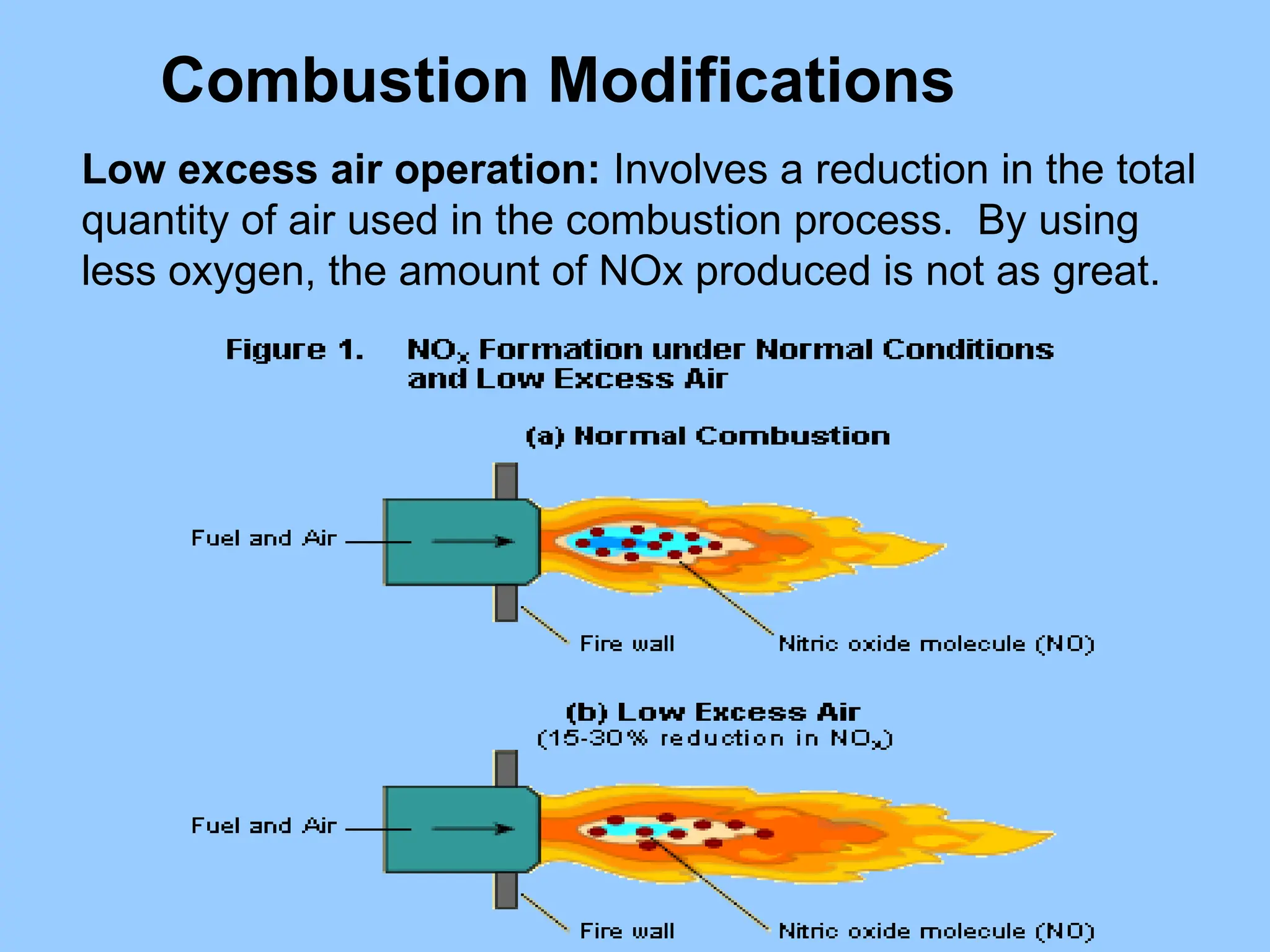
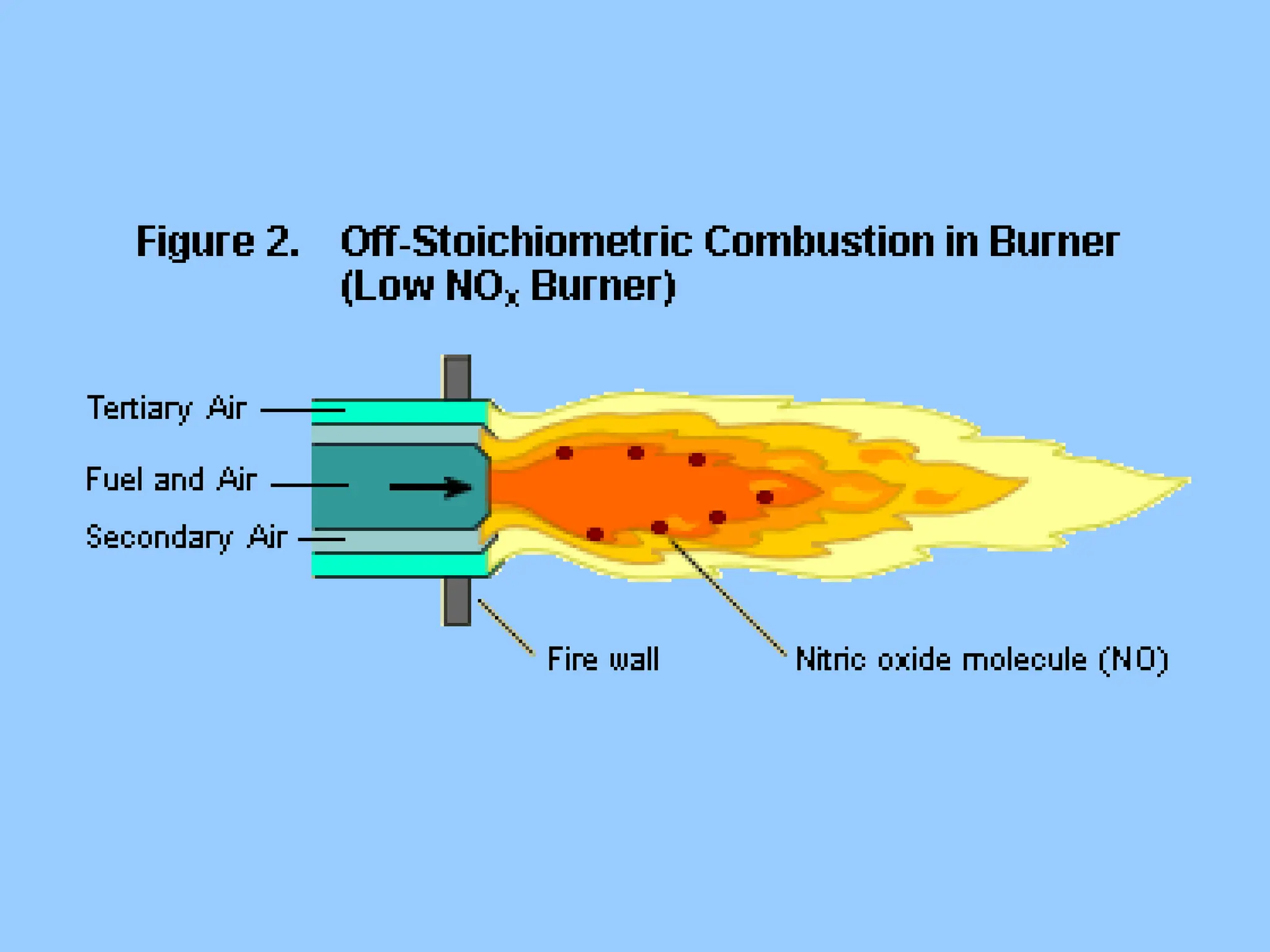
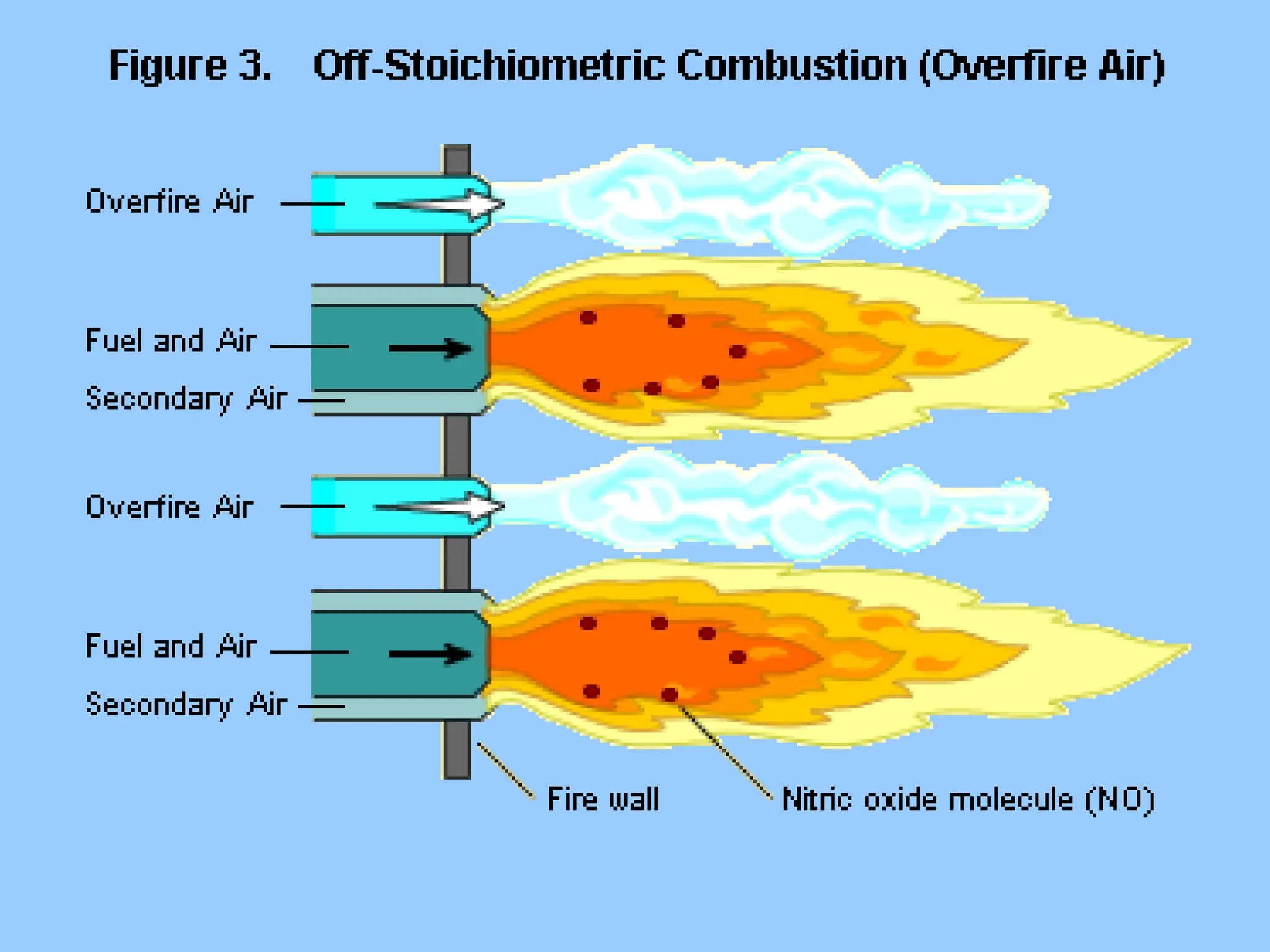
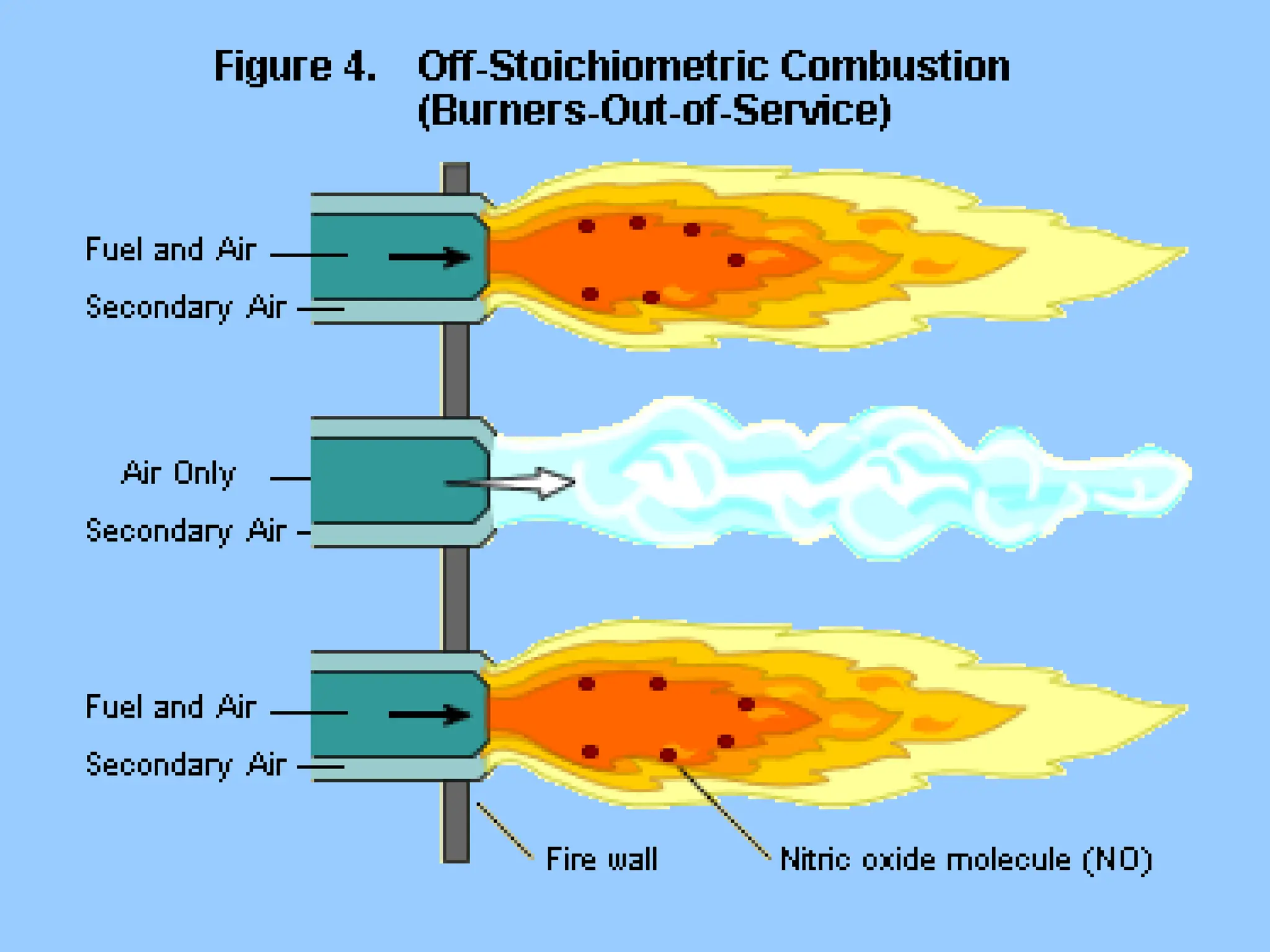
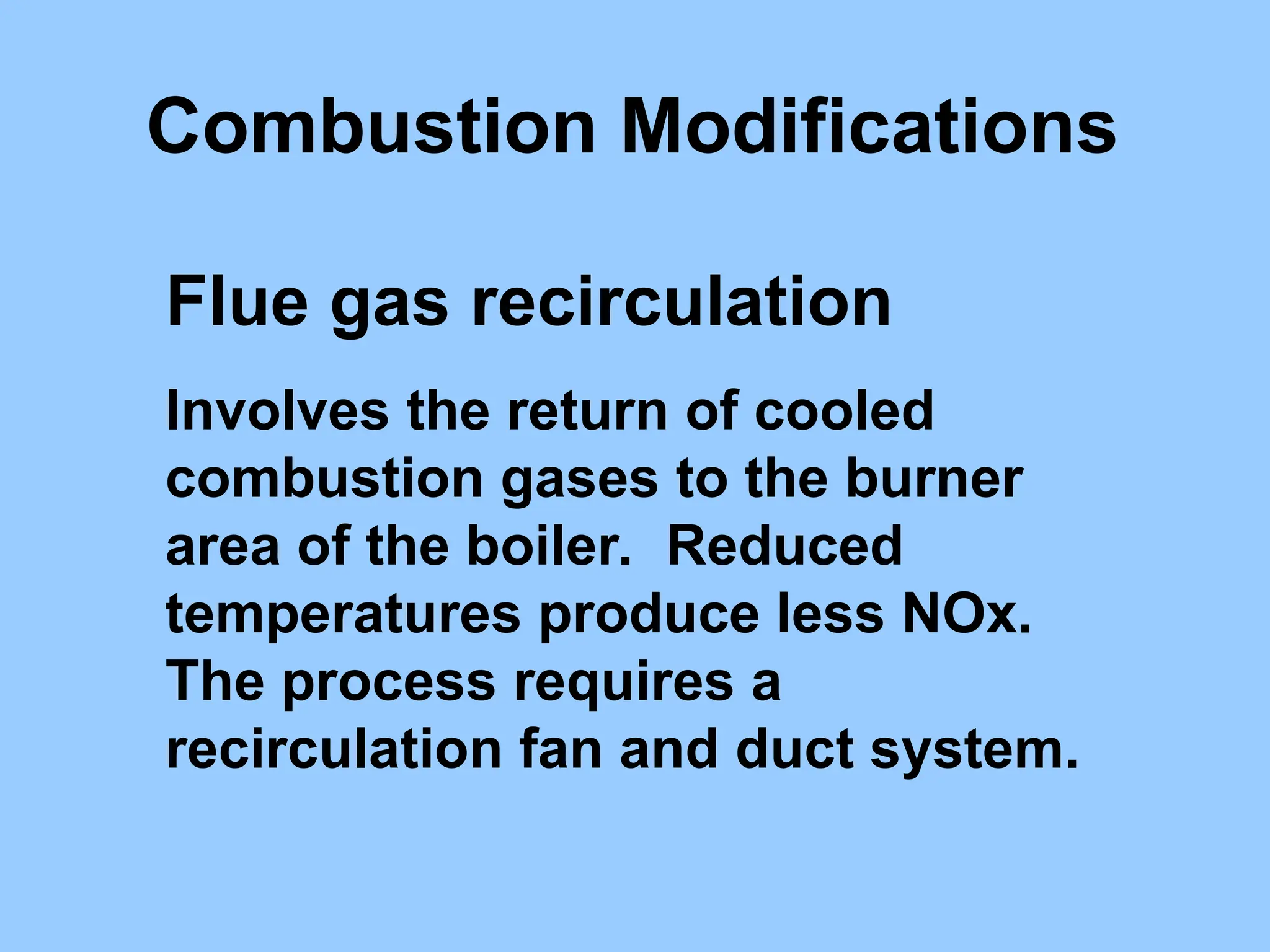
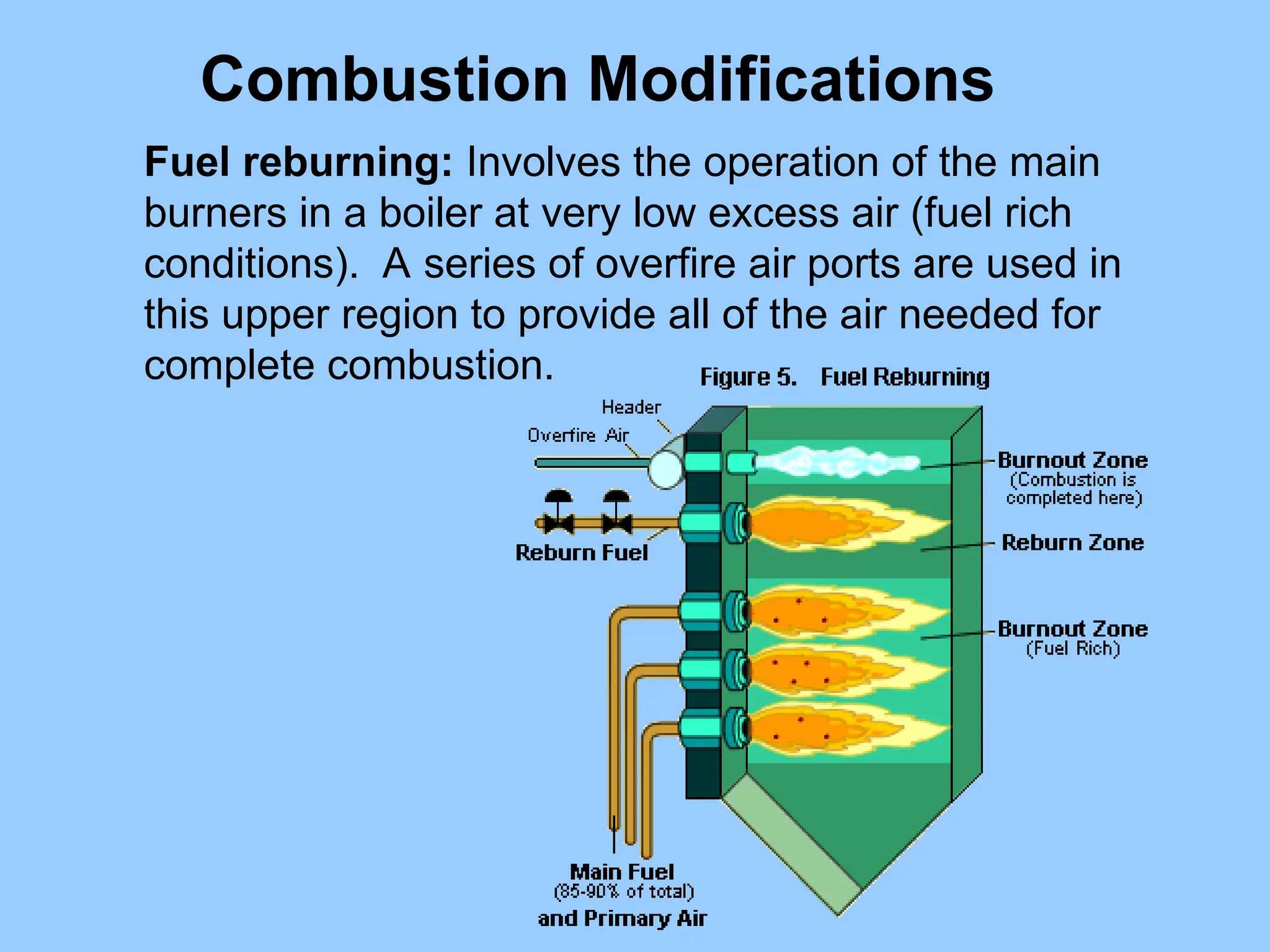
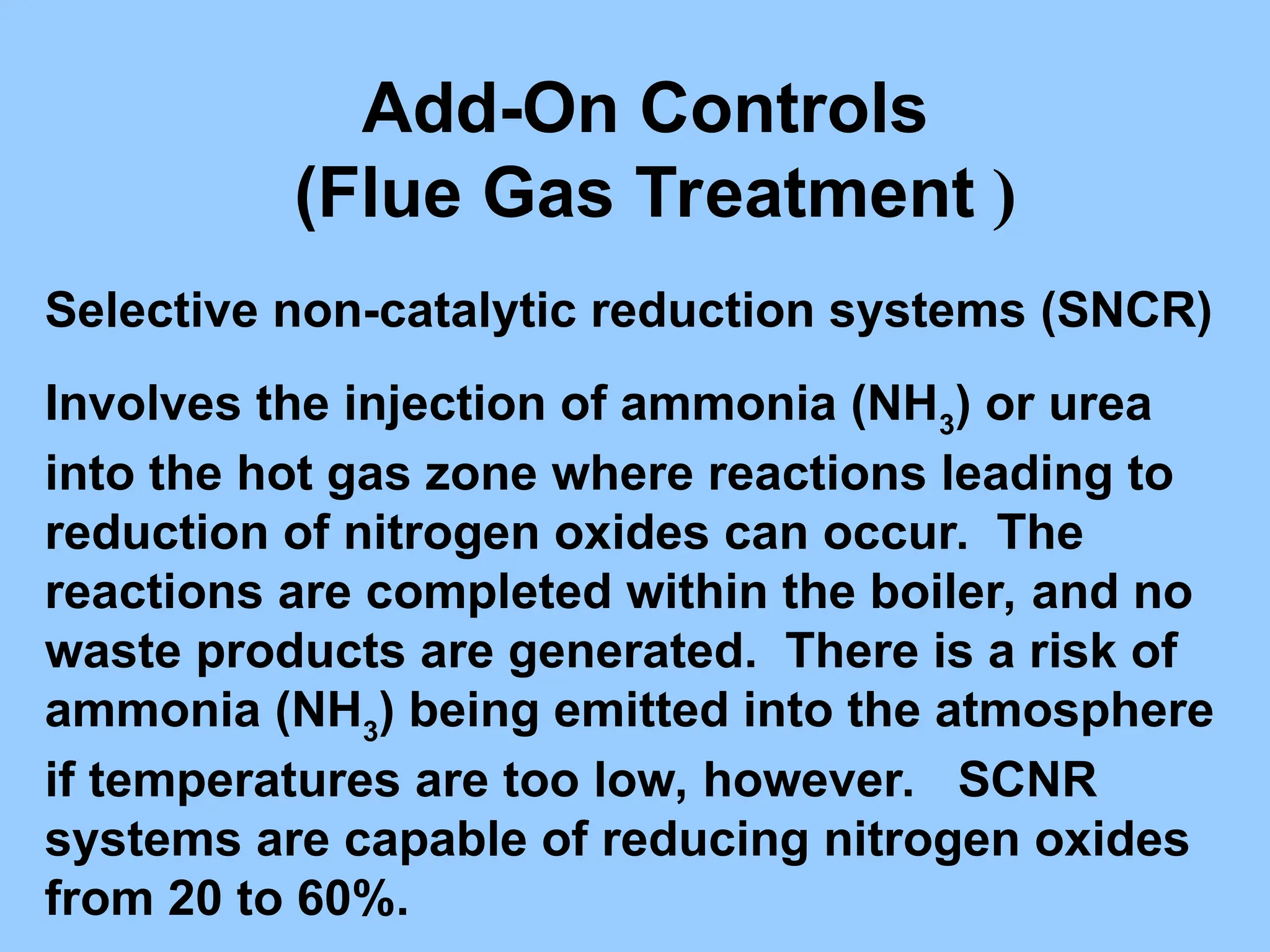
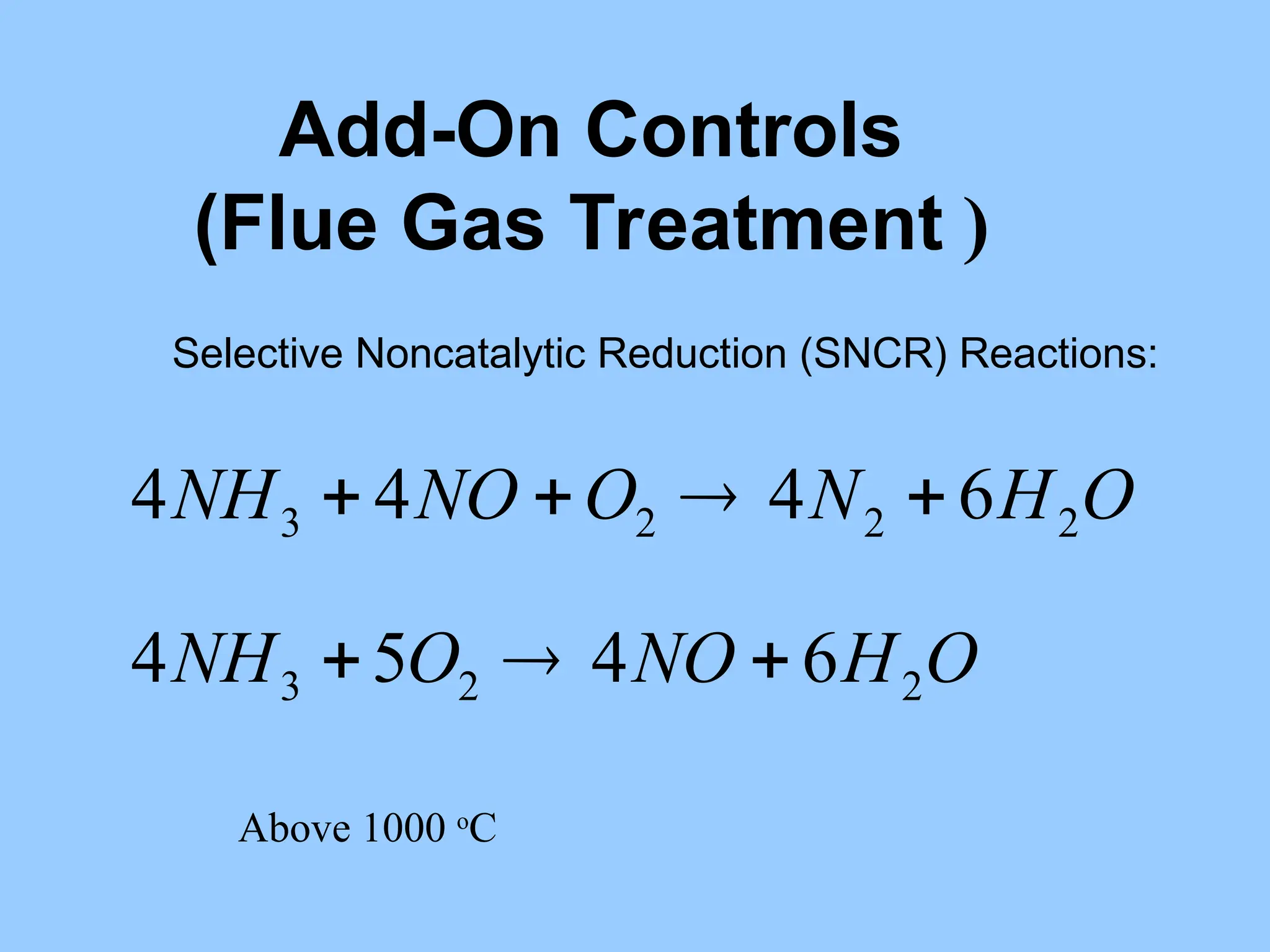
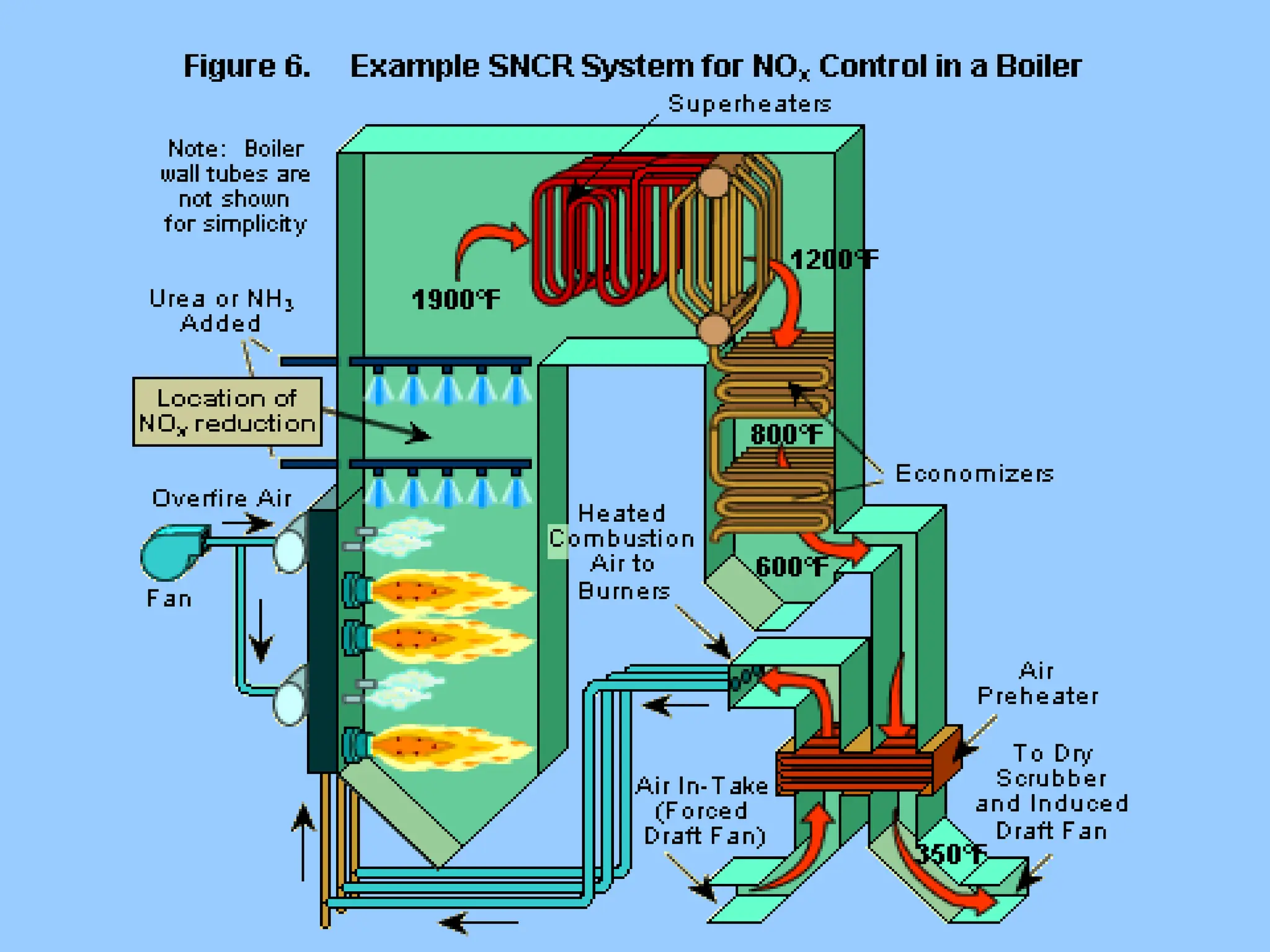
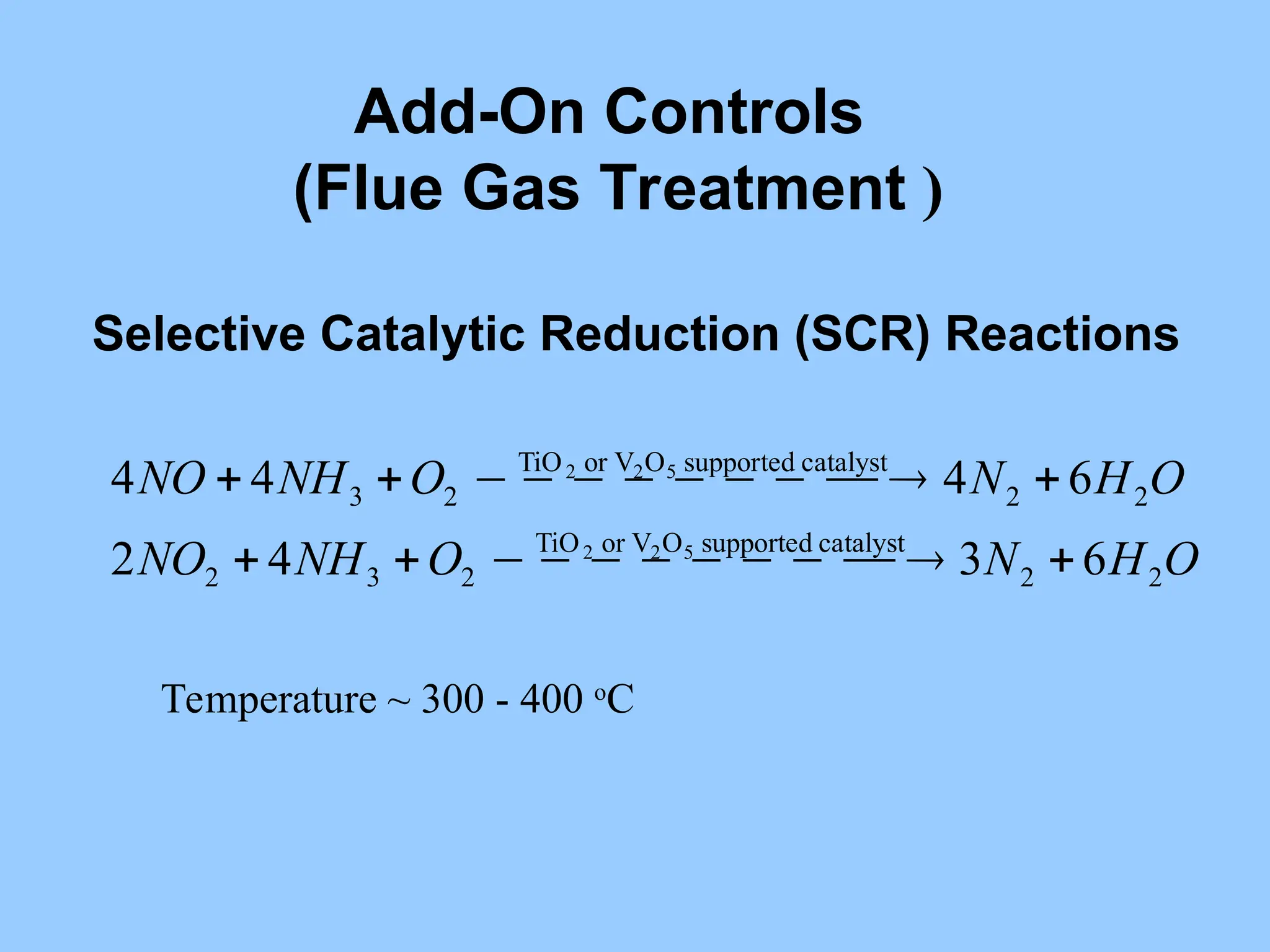
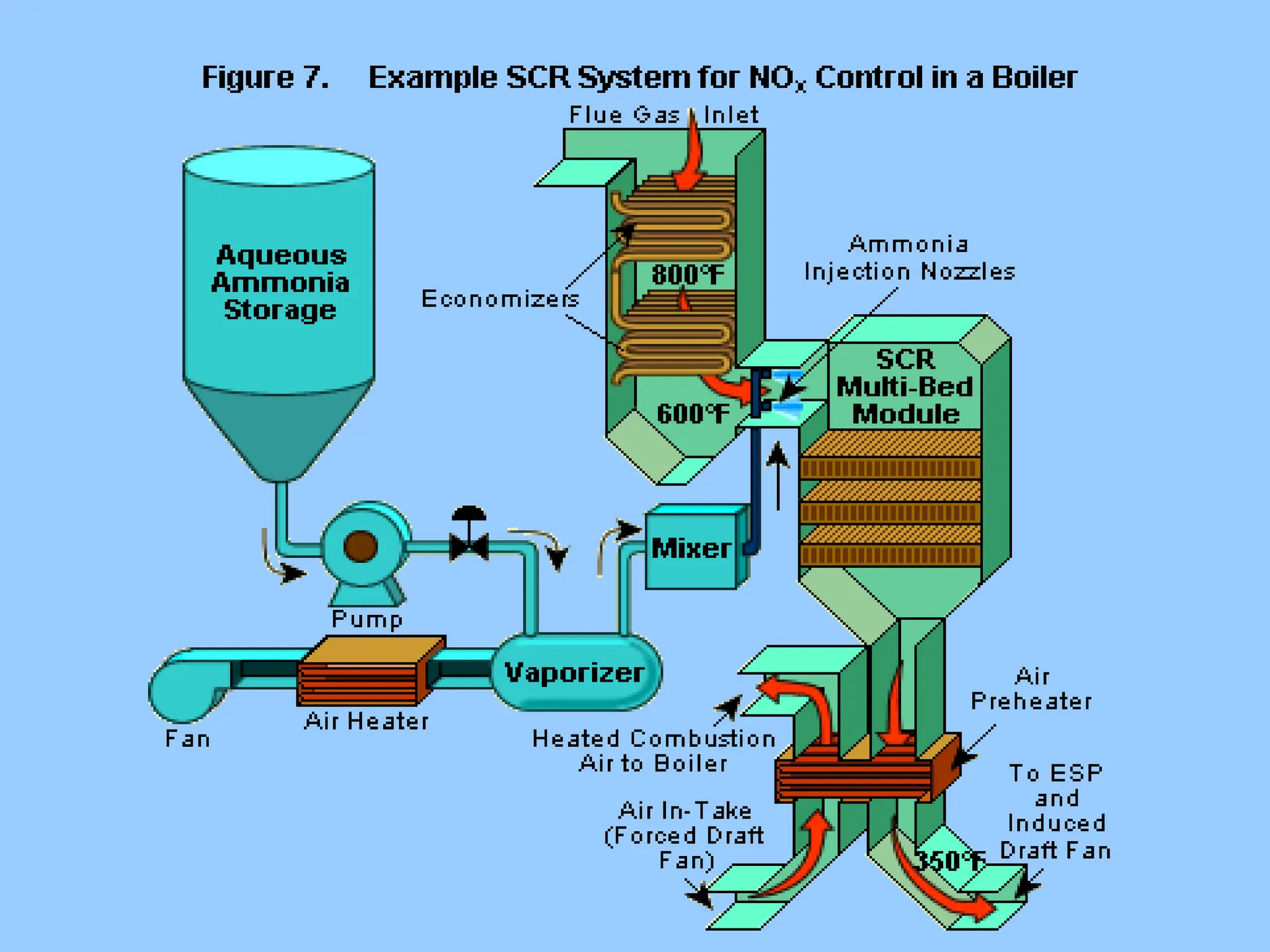
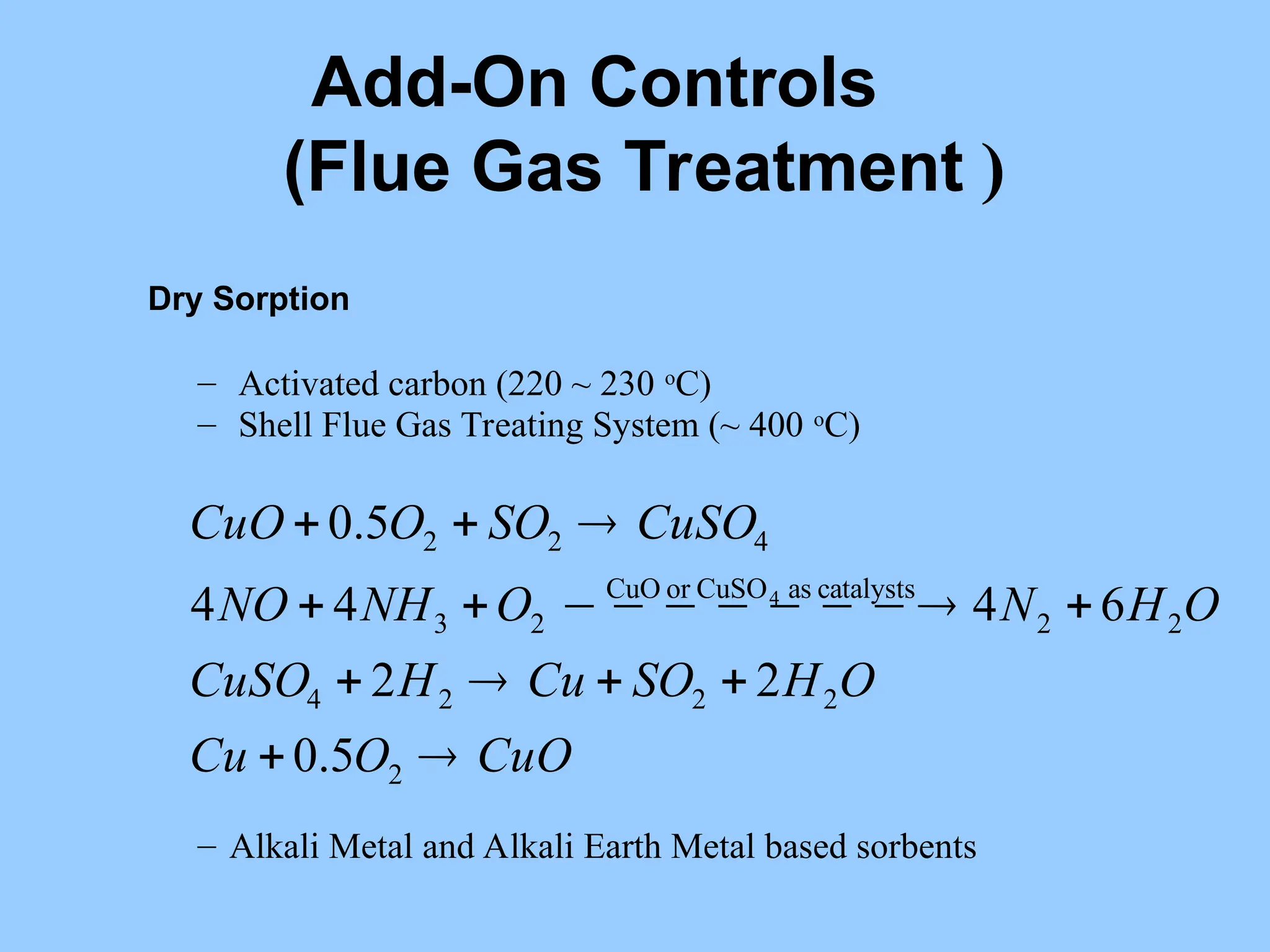
The document discusses sources of nitrogen oxides (NOx), such as combustion processes from vehicles and industrial operations, and their environmental impacts including respiratory issues and contribution to acid rain. It outlines various NOx control technologies, including modifications in combustion processes and flue gas treatment methods like selective catalytic reduction (SCR) and selective non-catalytic reduction (SNCR). Additionally, it highlights that conventional wet scrubbers are ineffective for NOx control due to the low solubility of NO in water.