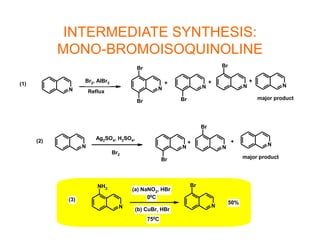
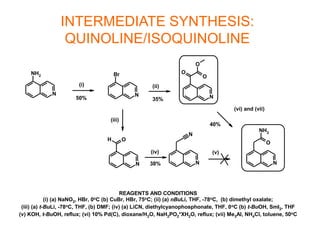
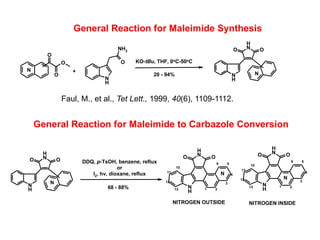
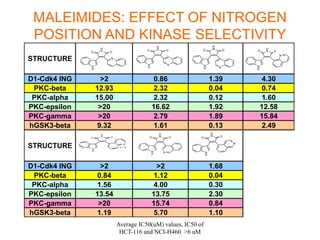
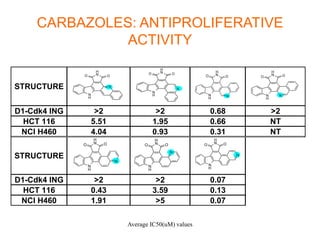
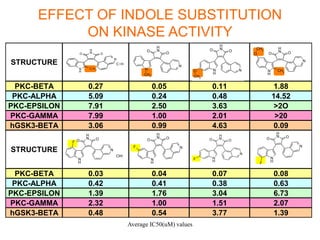
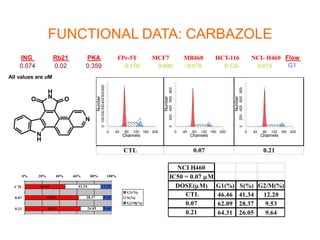
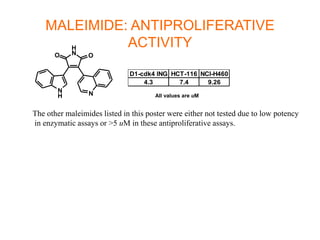
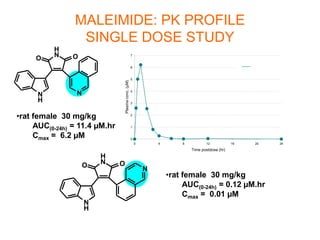
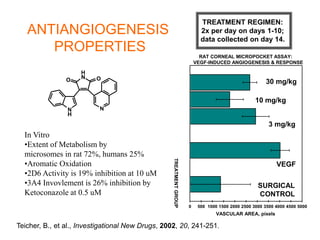
The document describes the synthesis and biological evaluation of novel indolyl-isoquinolinyl/quinolinyl-pyrrole-2,5-diones and corresponding carbazole analogs as potential kinase inhibitors. The maleimide analogs showed potent and selective inhibition of PKC isoforms but weaker activity against D1-cdk4. In contrast, the carbazole analogs were potent and selective D1-cdk4 inhibitors with anticancer activity in human tumor cell lines. However, the maleimides displayed poor pharmacokinetic properties that may have limited their in vivo activity. Structure-activity relationships revealed the importance of nitrogen position in the isoquinoline ring for PKC selectivity and potency