The document describes the synthesis and evaluation of 6,7-dihydro-4H-isothiazolo[4,5-b]pyridin-5-ones (DIP) as potential antimitotic agents. A series of DIP derivatives were synthesized via a multicomponent reaction. Selected DIP derivatives were found to alter sea urchin egg cleavage at low nanomolar concentrations and showed cytotoxicity against cancer cells, including chemoresistant lines, at submicromolar to low nanomolar levels. Both the sea urchin embryo assay and cancer cell assays confirmed the DIP derivatives act by destabilizing microtubules. Structure-activity relationship studies showed the importance of a p
![Accepted Manuscript
Synthesis and anti-mitotic activity of 6,7-dihydro-4H-isothiazolo[4,5-b]pyridin-5-ones:
In vivo and cell-based studies
Victor V. Semenov, Boris V. Lichitsky, Andrey N. Komogortsev, Arkady A. Dudinov,
Mikhail M. Krayushkin, Leonid D. Konyushkin, Olga P. Atamanenko, Irina B.
Karmanova, Yuri A. Strelenko, Boris Shor, Marina N. Semenova, Alex S. Kiselyov
PII: S0223-5234(16)30811-X
DOI: 10.1016/j.ejmech.2016.09.075
Reference: EJMECH 8941
To appear in: European Journal of Medicinal Chemistry
Received Date: 7 May 2016
Revised Date: 26 June 2016
Accepted Date: 23 September 2016
Please cite this article as: V.V. Semenov, B.V. Lichitsky, A.N. Komogortsev, A.A. Dudinov,
M.M. Krayushkin, L.D. Konyushkin, O.P. Atamanenko, I.B. Karmanova, Y.A. Strelenko, B.
Shor, M.N. Semenova, A.S. Kiselyov, Synthesis and anti-mitotic activity of 6,7-dihydro-4H-
isothiazolo[4,5-b]pyridin-5-ones: In vivo and cell-based studies, European Journal of Medicinal
Chemistry (2016), doi: 10.1016/j.ejmech.2016.09.075.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to
our customers we are providing this early version of the manuscript. The manuscript will undergo
copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please
note that during the production process errors may be discovered which could affect the content, and all
legal disclaimers that apply to the journal pertain.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-1-320.jpg)

![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
1
Synthesis and anti-mitotic activity of 6,7-dihydro-4H-isothiazolo[4,5-b]pyridin-5-ones:
in vivo and cell-based studies
Victor V. Semenov a,
*, Boris V. Lichitsky a
, Andrey N. Komogortsev a
, Arkady A. Dudinov a
,
Mikhail M. Krayushkin a
, Leonid D. Konyushkin a
, Olga P. Atamanenko a
, Irina B. Karmanova a
,
Yuri A. Strelenko a
, Boris Shor b
, Marina N. Semenova c,d
, Alex S. Kiselyov e
a
N. D. Zelinsky Institute of Organic Chemistry, RAS, Leninsky Prospect, 47, 119991, Moscow,
Russian Federation
b
Immune Pharmaceuticals LLC, 430 East 29th Street, Suite 940, New York, NY, 10016, USA
c
N. K. Kol’tsov Institute of Developmental Biology, RAS, Vavilov Street, 26, 119334, Moscow,
Russian Federation
d
Chemical Block Ltd., 3 Kyriacou Matsi, 3723, Limassol, Cyprus
e
Life Sciences Center, Moscow Institute of Physics and Technology, Institutsky Per., 9,
Dolgoprudny, Moscow Region, 141700, Russian Federation
Corresponding author: Victor V. Semenov
Address: N. D. Zelinsky Institute of Organic Chemistry, RAS, Leninsky Prospect, 47, 119991,
Moscow, Russian Federation. Tel.: +7 916 620 9584; fax: +7 499 137 2966.
E-mail: vs@zelinsky.ru
E-mail addresses:
Victor V. Semenov vs@zelinsky.ru
Boris V. Lichitsky blich2006@mail.ru
Andrey N. Komogortsev dna5@mail.ru
Arkady A. Dudinov 1944ark@mail.ru
Mikhail M. Krayushkin mkray@ioc.ac.ru
Leonid D Konyushkin LeonidK@chemical-block.com
Olga P. Atamanenko info@chemblock.com
Irina B. Karmanova vs@chemblock.ru
Yuri A. Strelenko strel@ioc.ac.ru
Shor Boris boris.shor@immunepharma.com
Marina N. Semenova ms@chemical-block.com
Alex S. Kiselyov nikizalp@aol.com](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-3-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
2
ABSTRACT
A series of 3,7-diaryl-6,7-dihydroisothiazolo [4,5-b]pyridin-5(4H)-ones 8 and 9 was synthesized by
multicomponent condensation of 3-aryl-5-isothiazolecarboxylic acid esters 4a–f with aromatic (or
thienyl) aldehydes 7 and Meldrum's acid in an acidic medium. The targeted compounds were
evaluated for their antimitotic microtubule destabilizing activity using in vivo phenotypic sea urchin
embryo model and in vitro human cancer cell-based assays. Selected dihydroisothiazolopyridinones
altered sea urchin egg cleavage in 2–10 nM concentrations together with significant cytotoxicity
against cancer cells including chemoresistant cell lines (IC50 in submicromolar – low nanomolar
concentration range). Both approaches confirmed antimitotic microtubule destabilizing mechanism
of action of the izothiazole derivatives. Structure-activity relationship study determined the
importance of p-methoxybenzene A-ring for the antiproliferative effect. The most potent compound
9b containing p-methoxybenzene A-ring and thiophene B-ring caused mitotic arrest and
disintegration of cell microtubules.
Keywords:
Isothiazoles
Dihydroisothiazolopyridinones
Microtubule destabilization
Sea urchin embryo
Cytotoxicity
Abbreviations:
CA4P, combretastatin A-4 disodium phosphate
DIP, 6,7-dihydro-4H-isothiazolo[4,5-b]pyridin-5-ones
MDR, multi-drug resistance
Pgp, P-glycoprotein
SAR, structure-activity relationship](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-4-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
3
1. Introduction
Derivatives of 4-aminoisothiazole exhibit a diverse range of pharmacological and biological
activities. In a few representative examples, 3-(hetero)aryl-4-aminothiazoles were described as
potent ATP-competitive inhibitors of kinases including vascular endothelial growth factor receptors
I and II [1] and cyclin-dependent kinases [2]. Lysophosphatic acid receptor antagonists based on this
chemotype has been reported [3]. Heterobiaryl compounds containing 4-aminoisothiazole moiety
(Fig. 1, I) showed potent antimitotic microtubule destabilizing activity [4]. Related derivatives
containing cyclic amide group were introduced recently (Fig. 1, II) [5], however their biological
activity was not described. It is reasonable to assume that proper substitution of rings A and B in II
may yield novel potent tubulin/microtubule targeting agents [4]. In this work an optimized path
towards 4-aminoisothiazole derivatives II has been devised. The targeted compounds were
evaluated as microtubule destabilizing agents using in vivo phenotypic sea urchin embryo assay and
in vitro cell-based approaches.
N
H
N
S
O
II
R1
R2
R1= H, Cl
R2= H, OMe, Hal
NH N
N
O
NH
N
S
Py
(OCH3)n
I B
A
Fig. 1. Structures of 4-aminoisothiazole derivatives.
2. Results and discussion
2.1. Chemistry
In order to develop a general approach to synthesis of the targeted 3,7-diaryl-6,7-
dihydroisothiazolo [4,5-b]pyridin-5(4H)-ones (II), we turned our attention to the expedited
preparation of the key 4-aminoisothiazoles 6a–f from the easily available benzonitriles 1a–f via the
described reaction sequence [6]. It involved generation of oximes 2a–f from 1 followed by their
interaction with TsCl and subsequent cyclization of tosylates 3a–f with thioacetic ester to arrive at
3-aryl-5-isothiazolecarboxylic acid esters 4a–f [6]. Reflux of 4a–f in concentrated HCl afforded
intermediate acids 5a–f followed by their subsequent decarboxylation to result in 4-
aminoisothiazoles 6a–f, generated in quantitative yields as respective HCl salts and used without
further separation in the next step. A multicomponent condensation of 6a–f with aromatic aldehydes
7 and Meldrum's acid in a glacial AcOH/AcONa system afforded the desired 6,7-dihydro-4H-](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-5-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
4
isothiazolo[4,5-b]pyridin-5-ones (DIP) 8, 9 (35–65% overall yield starting from 4). Aldehydes 7k,
7l, 7m (myristicin-, dillapiol-, apiol-derivatives, respectively), and 7n were prepared using the
essential oils isolated from dill and parsley seeds, as per conversion routes described earlier [7,8].
a
1a-f 4a-f2a-f
N
AX AX
3a-f
N
N
OTs
AX
5a-f
N
S
NH2
O
OH
AX
N
S
NH2
O
H3CO
AX
HSCH2CO2CH3
b c
X
a: Í
b: 4-OCH3
c: 3,4-(OCH3)2
d: 4-Cl
e: 3-F
f: 4-F
d
OO
O O
S
O
6a-f
47-62 %
9b,d-f
N
S
N
H
O
R1
R2R3
R4
X A
B
7g-r
R1
R2
R3
R4
O
B
N
S
N
H
O
S
AX
ff
R1 R2, R3 R4
g: Í H OCH3 H
h: H OCH3 H H
i: H -OCH2O- H
j: H -OCH2CH2O- H
k: H OCH3 -OCH2O-
l: OCH3 OCH3 -OCH2O-
m: OCH3 -OCH2O- OCH3
n: OCH3 OH OH OCH3
o: H Cl H H
p: H H Cl H
q: H F H H
r: H H F H
8a(k,m,n), 8b(h-r), 8co,
8d(k,m), 8e(g-i,k,m,o-r),
8f(g-i,k,m,o,r)
e
35-65 %
Na
+
N
N
O
-
N
S
NH3
+
X A
Cl
-
Scheme 1. Reagents and conditions: (a) NaOMe–i-AmylONO, abs. MeOH, 5 °C, 1 h [9]; (b) TsCl,
DMF, 75 °C, 15 min [10]; (c) HSCH2CO2Me, MeOH, Et3N, r.t., 4 h [6]; (d) and (e) conc. HCl,
reflux, 6 h; (f) NaOAc–AcOH, reflux, 2 h.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-6-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
5
2.2. Biological evaluation
2.2.1. Antimitotic activity in the sea urchin embryo model
The resulting DIP 8 and 9 were evaluated in a phenotypic sea urchin embryo assay for their
antimitotic microtubule destabilizing activity [11]. This assay system has been introduced and
extensively validated by our team to identify compounds that selectively affect tubulin dynamics.
We have consistently demonstrated excellent correlation between data generated using the in vivo
sea urchin embryo assay, in vitro tubulin polymerization, and human tumor cell experiments for
diverse chemical series [4,12,13]. Specifically, the assay includes monitoring of: i) cleavage
alteration and/or arrest of fertilized eggs to assess antimitotic activity, and ii) motility of free-
swimming blastulae exposed to a compound. Distinct swimming alterations, namely, settlement to
the bottom of a well accompanied by a rapid spinning around animal-vegetal axis, suggest
microtubule destabilizing mode of action of a tested molecule (video illustrations are available at
http://www.chemblock.com). The results are presented in Table 1, with combretastatin A-4
disodium phosphate (CA4P) as a positive control.
Table 1
Effects of 6,7-dihydro-4H-isothiazolo[4,5-b]pyridin-5-ones (DIP) on the sea urchin embryos and
human cancer cell lines [14] (NCI60 anticancer drug screen).
N
S
N
H
O
R1
R2
3
7 6
Compd R1 R2 Sea urchin embryo effects, EC
(µM)a NCI60 screen
Cleavage
alteration
Cleavage
arrest
Embryo
spinning
Mean GI50,
µMb Mean GI, %c
CA4P 0.005 0.01 0.05 0.00171
8ak 4 >4 >4 4.6
8am >4 >4 >4 NDd
NDd
8an >4 >4 >4 NDd
NDd
8bh 0.01 0.05 0.1 1.549
O
O
OCH 3
O
O
OCH 3
OCH 3
OH
OH
OCH 3
OCH 3
OCH 3
OCH 3](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-7-320.jpg)

![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
7
8eo 1 4 >4 NDd
NDd
8ep >4 >4 >4 16.5
8eq 1 >4 >4 6.4
8er 1
4 (reverse,
TEf
)
>4 NDd
NDd
8fg 2 >4 >4 6.2
8fh 1
4 (reverse,
TEf
)
>4 15.1
8fi 1 >4 >4 11.7
8fk >4 >4 >4 10.1
8fm >4 >4 >4 NDd
NDd
8fo 1 4 >4 15.6
8fr 1 4 (reverse) >4 4.2
9b 0.002 0.005 0.1 0.302
9d 0.01 0.1 0.2 1.514
9e 0.05 0.5 2 7.8
9f 0.05 0.2 4 NDd
NDd
a
The sea urchin embryo assay was conducted as described previously [11]. Fertilized eggs and
hatched blastulae were exposed to 2-fold decreasing concentrations of compounds. Duplicate
measurements showed no differences in effective threshold concentration (EC) values.
b
GI50: concentration required for 50% cell growth inhibition.
c
GI %: single dose inhibition of cell growth at 10 µM concentration of a compound.
d
ND: not determined.
e
Due to incomplete solubility of compound 8bk in DMSO, approximate EC values are presented.
f
TE: tuberculate eggs typical of microtubule destabilizing agents.
F
F
Cl
F
F
OCH 3
F
F
F
F
F
F
OCH 3
O
O
O
O
OCH 3
O
O
OCH 3
OCH 3
Cl
F
S
S
S
SF
F
Cl
OCH 3
F Cl
F
F](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-9-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
8
As shown in Table 1, compounds 8bh, 8bi, 8bj, 8bm, 8bo, 8bp, 8bq, 8br, 9b, 9d, 9e, and 9f
potently inhibited cell division, resulting in cleavage alteration, cleavage arrest and embryo spinning
suggestive of their specific tubulin targeting and microtubule destabilizing activities [11]. Whereas
DIP 8bl, 8er, and 8fh failed to induce embryo spinning, these molecules did trigger the formation of
tuberculate-shaped arrested eggs specific for microtubule destabilizers [11]. We reasoned that
compounds 8bl, 8er, and 8fh could be considered as less active microtubule destabilizing agents in
the sea urchin embryo assay. Notably, compounds 8bn, 8co, 8dk, 8eh, 8ei, 8eo, 8fg, 8fi, and 8fo
exhibited non-tubulin antiproliferative activity with morphogenetic abnormalities at post-hatching
stages. These included developmental delay and inhibition of skeletal spicule growth resulting in the
formation of aberrant pluteus larvae. Compounds 8fk and 8fm failed to affect cleavage up to 4 µM
concentration. Both molecules did inhibit growth of skeletal spicules at the early pluteus stage (1
µM concentration). It should be noted that several molecules exhibited limited solubility in the
seawater. Specifically, formation of microcrystals was observed using light microscope for
compounds 8bi (at 1 µM), 8bh (at 2 µM) 8dk, 8eh, 8ep and 8fr (at 4 µM). DIP 8bk had marginal
solubility in DMSO even at heating, therefore its effective concentrations could not be estimated
accurately.
The structure-activity relationship (SAR) study showed that antimitotic anti-tubulin effects
of DIP 8 and 9 were associated with the substitution pattern in the A- and B-rings. As shown in the
Table 1, DIP containing p-methoxy substituent in the A-ring 8bh, 8bi, 8bj, 8bo, 8bq, 8br, and 9b
showed the highest activity (Table 1). Their effect strongly depended on the B-ring pharmacophore
pattern, the most active compound being 3-thiophene derivative 9b. Specific modifications of the
substitution pattern of the B-ring dramatically affected activities of the resulting molecules as well.
The following order of decreasing anti-tubulin activity of molecules with benzene B-ring was
observed: most active compounds m-OCH3 8bh = methylenedioxy 8bi = ethylenedioxy 8bj = m-Cl
8bo = m-F 8bq = p-F 8br > apiol-derived 8bm > p-Cl 8bp > dillapiol-derived 8bl > o,m-dimethoxy-
m,p- dihydroxy 8bn. The last molecule was the only one with the p-methoxy substituted A-ring that
exhibited non-tubulin antiproliferative effect. Introduction of four substituents into the B-ring led to
less active molecules.
Several modifications of the A-ring were found to decrease antiproliferative activity.
Namely, introduction of additional methoxy group reduced activity (compare 8bo vs 8co). Similarly,
removal of p-methoxy group from the A-ring of compound 8bn yielded inactive 8an. Compound
8ak with unsubstituted A-ring and myristicin-derived B-ring exhibited low activity. In a series of
DIP featuring apiol-derived B-ring (8bm, 8dm, 8em, and 8fm), 8bm substituted with p-methoxy
group in the A-ring was discovered to be the most potent molecule. Removal of p-methoxy group
(8am) or its replacement with p-Cl (8dm), m-F (8em), or p-F (8fm) resulted in significant loss of](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-10-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
9
activity. Considerable decrease of antimitotic effect was also observed for the replacement of p-
methoxy group in the A-ring with m-F or p-F moiety (compare 8bh vs 8eh and 8fh; 8bi vs 8ei and
8fi; 8bo vs 8eo and 8fo; 8bq vs 8eq; 8br vs 8er and 8fr). Compounds with m-Cl substituted B-ring
(8co, 8eo, and 8fo) showed potential tubulin/microtubule-independent antiproliferative effect on the
sea urchin embryos as well as developmental abnormalities after hatching. Noteworthy, all DIP that
contained thiophene B-ring showed significant antimitotic microtubule destabilizing activity
including molecules 9d, 9e, and 9f with p-Cl-, m-F-, and p-F-substituted A-rings, respectively. Their
potency decreased in the following order of the A-ring substitution: m-OCH3 9b > m-F 9e = p-F 9f >
p-Cl 9d. Interestingly, thiophene-based combretastatin analogues have been reported recently as
anti-tubulin agents [15].
The sea urchin embryos were found to be more sensitive towards DIP, than cancer cells. A
possible explanation for this observation is that, on average, it takes 20–24 h for the mitotic cycle of
cultured cancer cells to complete, whereas the sea urchin embryo blastomeres divide every 35–40
min. This longitudinal difference may result in antitubulin agents to interact predominantly with
interphase microtubules in cancer cells and to affect specifically microtubules of mitotic spindle in
the sea urchin embryo. Nevertheless, the data of the sea urchin embryo assay correlated well with
the NCI60 anticancer drug screen results (Table 1). The most potent compound 9b identified in the
sea urchin embryo model exhibited the highest cytotoxicity in the panel of cancer cell lines as well.
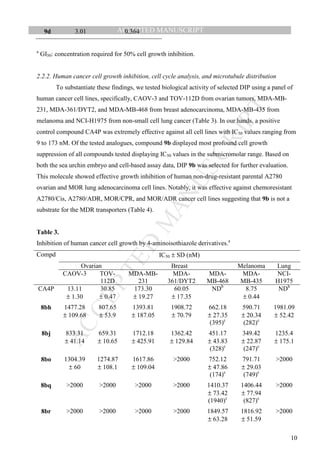
Tubulin/microtubule-targeting compounds 8bh, 8bi, 8bj, 8bo, 8bq, 8br, and 9d that markedly
altered cleavage, inhibited growth of human cancer cells with GI50 in low micromolar
concentrations. Identified active DIP 8bh, 8bj, 8bo, 8bq, 8br, and 9d inhibited growth of ovarian P-
glycoprotein (Pgp)-overexpressing multi-drug resistant (MDR) cell line NCI/ADR-RES (Table 2),
suggesting that these compounds were not Pgp substrates.
Table 2.
Growth inhibition of OVCAR-8 ovarian cancer cells and related multi-drug resistant NCI/ADR-RES
cells by DIP 8bh, 8bi, 8bj, 8bo, 8bq, 8br, and 9d in NCI60 screen.
Compd NCI60 screen, GI50 (µM)a
OVCAR-8 NCI/ADR-RES
8bh 3.22 0.42
8bi 3.04 0.524
8bj 1.41 0.367
8bo 2.92 0.562
8bq 7.54 2.96
8br 4.01 2.11](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-11-320.jpg)

![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
12
MB-468 also produced a large increase in sub-G1 population that was associated with the reduction
of G1 and S phase populations. Considerable differences in the doubling time of NCI-H1975 vs
MDA-MB-468 (21 h vs 62 h, respectively [16,17]) as well as distinct mutational background of both
cell lines (MDA-MB-468 is Rb-negative line) could provide one potential explanation for the
observed cell line-specific outcome in the analysis. Overall, these results support the hypothesis that
compound 9b elicited its biological effects by inducing cell cycle arrest at the mitotic phase with
further initiation of apoptotic cell death.
Fluorescent microscopy using an anti-tubulin antibody showed a disintegration of
microtubule network with more intense staining at cell periphery and lesser cytoplasmic volume in
NCI-H1975 cells treated with 1µM of 9b for 16 or 24 h (Fig. 4). Collectively, our findings
demonstrated that antiproliferative effects observed with DIP 9b in human cancer cells could be
accounted for, at least in part, by the marked mitotic arrest and pronounced loss of microtubule
integrity.
Fig. 2. Effect of compound 9b on mitotic index. NCI-H1975 or MDA-MB-468 cells were treated
with 0.1% DMSO (Control) or with 1 µM 9b for 24 h. Mitotic index (M) was determined by flow](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-14-320.jpg)

![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
14
and 24 h. Microtubules (red) were visualized with anti-β-tubulin antibody and Alexa Fluor 647
secondary antibody. Hoechst counterstain was performed to identify nuclei (blue). Representative
images are shown for each condition.
3. Conclusions
A series of 3,7-diaryl-6,7-dihydroisothiazolo [4,5-b]pyridin-5(4H)-ones 8 and 9 was
synthesized by multicomponent condensation of 3-aryl-5-isothiazolecarboxylic acid esters 4a–f with
aromatic aldehydes 7 and Meldrum's acid. A number of targeted compounds were found to affect
cell division through microtubule destabilizing mode of action in nanomolar concentrations as
evidenced from the phenotypic sea urchin embryo assay data. p-Methoxybenzene A-ring was
identified to be essential for the activity. Compound 9b with p-methoxybenzene A-ring and
thiophene B-ring showed the highest potency in both phenotypic sea urchin embryo and human
cancer cell cytotoxicity assays. Molecules 8bh, 8bi, 8bj, 8bo, 8bq, 8br, and 9d induced pronounced
growth inhibition of MDR cell lines. In the cell-based assays, compound 9b blocked cell cycle in
mitosis and disintegrated interphase microtubule network, suggesting its tubulin-targeting
microtubule destabilizing effect.
4. Experimental protocols
4.1. Chemistry. Materials and methods
Melting points were measured on a Boetius melting point apparatus and were uncorrected.
Reaction mixtures were stirred magnetically. 1
H NMR spectra were recorded on a Bruker DRX-500
(500.13 MHz) instrument. 13
C NMR spectra were recorded on a Bruker DRX-500 (125.8 MHz)
instrument. Chemical shifts are stated in parts per million and referenced to TMS and were assigned
as C, CH, CH2, and CH3 as determined using HSQC and HMBC 2D NMR experiments, where
necessary. Spin–spin coupling constants (J) were reported in hertz (Hz). An original software for
NMR spectra presentation in Supplementary data was designed at N. D. Zelinsky Institute of
Organic Chemistry (http://nmrix.ioc.ac.ru:8080).
Low resolution mass spectra (m/z) were recorded on a Finnigan MAT/INCOS 50 mass
spectrometer at 70 eV using direct probe injection. Elemental analysis was performed on the
automated PerkinElmer 2400 CHN microanalyzer. Flash chromatography was carried out on silica
gel (Acros, 0.035–0.070 mm, 60 Å). TLC was performed on Merck 60 F254 plates. Non-anhydrous
solvents and all reagents were purchased at the highest commercial quality and used as received.
The starting materials phenylacetonitriles 1a–f and benzaldehydes 7g–j, o–r were purchased from](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-16-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
15
Acros Organics (Belgium). Benzaldehydes 7k–m were synthesized from essential oils of parsley
and dill seeds according to published procedure [8]. The starting methyl 3-aryl-5-
isothiazolecarboxylates 4a–f were obtained using a method described in the literature[6].
4.1.1. Synthesis of 3,4-dihydroxy-2,5-dimethoxybenzaldehyde (7n)
The mixture of apiolaldehyde 7m (21 g, 0.1 mol), PCl5 (62.5 g, 0.3 mol), and CHCl3 (10 mL)
was boiled at 98–100 °C for 5 h, then concentrated in rotary evaporator, diluted with water (100 mL)
and kept overnight. Water suspension was boiled for 3 h, the resulting dark solution cooled to 0–5
°C. The precipitate was filtered, washed several times with water, and dried to afford benzaldehyde
7n. Yield 87%; dark crimson solid; mp 124–127 °C.
4.1.2. General procedure for the synthesis of 3,7-diaryl-6,7-dihydro-4H-isothiazolo[4,5-b]pyridin-5-
ones (8, 9)
Methyl 3-aryl-5-isothiazolecarboxylates 4a–f (2 mmol) were refluxed in 15 ml of
concentrated HCl for 6 h. The resulting reaction mixture was concentrated in vacuo. Anhydrous
NaoAc (0.18 g, 2.2 mmol), Meldrum's acid (0.32 g, 2.2 mmol), the corresponding aldehyde 7 (2.2
mmol), and glacial AcOH (7 mL) were added, the resulting suspension was heated at reflux for 2 h
and concentrated. The residue was recrystallized from the aqueous EtOH (60%), washed with
aqueous EtOH and dried to afford analytically pure compounds 8 and 9 (35–65% overall yields from
4a–f).
4.1.3. Characterization of compounds 8 and 9 (Table 1)
4.1.3.1. 6,7-Dihydro-7-(7-methoxy-1,3-benzodioxol-5-yl)-3-phenyl-isothiazolo[4,5-b]pyridin-5(4H)-
one (8ak). Yellowish solid, mp 190–192 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.81 (dd, J = 6.1
Hz, J = 15.6 Hz, 1H, CH2), 2.95 (dd, J = 10.0 Hz, J = 15.6 Hz, 1H, CH2), 3.82 (s, 3H, OCH3-7''),
4.63 (dd, J = 6.1 Hz, J = 10.0 Hz, 1H, CH), 5.99 (s, 2H, OCH2O), 6.61 (d, J = 1.6 Hz, 1H, H-4''),
6.70 (d, J = 1.6 Hz, 1H, H-6''), 7.49 (m, 3H, H-3',4',5'), 7.73 (d, J = 8.2 Hz, 2H, H-2',6'), 10.02 (s,
1H, NH). EIMS m/z 380 [M]+
(4), 178 (5), 161 (8), 147 (8), 135 (11), 121 (19), 120 (10), 107 (14),
104 (44), 103 (29), 89 (14), 77 (100), 63 (39). Anal. Calcd for C20H16N2O4S: C, 63.15; H, 4.24; N,
7.36. Found: C, 63.09; H, 4.22; N, 7.41.
4.1.3.2. 7-(4,7-Dimethoxy-1,3-benzodioxol-5-yl)-6,7-dihydro-3-phenyl-isothiazolo[4,5-b]pyridin-
5(4H)-one (8am). Yellowish solid, mp 184–185 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.87 (m, 2H,
CH2), 3.73 (s, 3H, OCH3-4''), 3.88 (s, 3H, OCH3-7''), 4.80 (t, J = 7.4 Hz, 1H, CH), 6.03 (s, 2H,](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-17-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
16
OCH2O), 6.48 (s, 1H, H-6''), 7.48 (m, 3H, H-3',4',5'), 7.72 (d, J = 8.2 Hz, 2H, H-2',6'), 10.00 (s, 1H,
NH). EIMS m/z 410 [M]+
(7), 229 (11), 182 (24), 167 (8), 161 (5), 147 (6), 135 (13), 121 (9), 109
(12), 104 (42), 103 (27), 95 (12), 93 (15), 77 (100), 63 (23). Anal. Calcd for C21H18N2O5S: C, 61.45;
H, 4.42; N, 6.83. Found: C, 61.53; H, 4.46; N, 6.74.
4.1.3.3. 7-(3,4-Dihydroxy-2,5-dimethoxyphenyl)-6,7-dihydro-3-phenyl-isothiazolo[4,5-b]pyridin-
5(4H)-one (8an). Yellowish solid, mp 253–255 °C (decomp.). 1
H NMR (DMSO-d6, 500 MHz): δ
2.77 (dd, J = 6.5 Hz, J = 15.7 Hz, 1H, CH2), 2.93 (dd, J = 9.8 Hz, J = 15.7 Hz, 1H, CH2), 3.66 (s,
3H, OCH3-2''), 3.72 (s, 3H, OCH3-5''), 4.77 (dd, J = 6.5 Hz, J = 9.8 Hz, 1H, CH), 6.35 (s, 1H, H-6''),
7.48 (m, 3H, H-3',4',5'), 7.72 (m, 2H, H-2',6'), 8.58 (br.s, 1H, OH-3''), 8.66 (br.s, 1H, OH-4''), 9.98
(s, 1H, NH). EIMS m/z 398 [M]+
(1), 229 (16), 170 (27), 155 (11), 149 (10), 135 (10), 121 (12), 109
(16), 104 (56), 103 (29), 95 (16), 77 (100). Anal. Calcd for C20H18N2O5S: C, 60.29; H, 4.55; N, 7.03.
Found: C, 60.20; H, 4.52; N, 7.12.
4.1.3.4. 6,7-Dihydro-7-(3-methoxyphenyl)-3-(4-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-
one (8bh). Yellowish solid, mp 168–170 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.86 (dd, J = 6.4
Hz, J = 15.6 Hz, 1H, CH2), 2.93 (dd, J = 9.0 Hz, J = 15.6 Hz, 1H, CH2), 3.75 (s, 3H, OCH3-3''), 3.82
(s, 3H, OCH3-4'), 4.69 (dd, J = 6.4 Hz, J = 9.0 Hz, 1H, CH), 6.88 (dd, J = 2.5 Hz, J = 8.3 Hz, 1H, H-
4''), 6.92 (m, 2H, H-2'',6''), 7.05 (d, J = 8.8 Hz, 2H, H-3',5'), 7.30 (t, J = 7.9 Hz, 1H, H-5''), 7.68 (d, J
= 8.8 Hz, 2H, H-2',6'), 10.02 (s, 1H, NH). 13
C NMR (DMSO-d6) δ 169.8, 160.0, 159.7, 156.4, 147.5,
142.5, 133.7, 130.1, 129.2, 126.2, 119.3, 114.1, 113.2, 112.8, 55.3, 55.1, 38.2, 37.6. EIMS m/z 366
[M]+
(73), 326 (5), 218 (11), 202 (28), 190 (14), 177 (10), 174 (10), 173 (14), 164 (23), 161 (12),
147 (12), 134 (71), 133 (39), 121 (43), 118 (23), 108 (19), 103 (49), 90 (86), 77 (94), 63 (97). Anal.
Calcd for C20H18N2O3S: C, 65.55; H, 4.95; N, 7.64. Found: C, 65.47; H, 4.91; N, 7.71.
4.1.3.5. 7-(1,3-Benzodioxol-5-yl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-
one (8bi). Yellowish solid, mp 176–178 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.80 (dd, J = 6.2 Hz,
J = 15.5 Hz, 1H, CH2), 2.90 (dd, J = 9.5 Hz, J = 15.5 Hz, 1H, CH2), 3.82 (s, 3H, OCH3-4'), 4.64 (dd,
J = 6.2 Hz, J = 9.5 Hz, 1H, CH), 6.01 (s, 1H, OCH2O), 6.02 (s, 1H, OCH2O), 6.81 (dd, J = 1.8 Hz, J
= 8.0 Hz, 1H, H-6''), 6.91 (d, J = 8.0 Hz, 1H, H-7''), 6.96 (d, J = 1.8 Hz, 1H, H-4''), 7.05 (d, J = 8.8
Hz, 2H, H-3',5'), 7.68 (d, J = 8.8 Hz, 2H, H-2',6'), 10.01 (s, 1H, NH). 13
C NMR (DMSO-d6) δ 169.9,
160.0, 156.4, 148.1, 147.7, 146.7, 134.8, 133.5, 129.1, 126.2, 120.5, 114.1, 108.5, 107.6, 101.2,
55.3, 38.4, 37.3. EIMS m/z 380 [M]+
(100), 365 (6), 337 (32), 322 (10), 259 (13), 246 (26), 217 (19),
190 (22), 178 (15), 161 (15), 147 (22), 134 (17), 120 (17), 90 (11), 89 (18), 77 (11), 63 (14). Anal.
Calcd for C20H16N2O4S: C, 63.15; H, 4.24; N, 7.36. Found: C, 63.07; H, 4.21; N, 7.42.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-18-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
17
4.1.3.6. 7-(2,3-Dihydro-1,4-benzodioxin-6-yl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-
b]pyridin-5(4H)-one (8bj). Yellow solid, mp 197–199 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.81
(dd, J = 6.5 Hz, J = 15.6 Hz, 1H, CH2), 2.86 (dd, J = 8.8 Hz, J = 15.6 Hz, 1H, CH2), 3.82 (s, 3H,
OCH3-4'), 4.23 (s, 4H, OCH2), 4.60 (dd, J = 6.5 Hz, J = 8.8 Hz, 1H, CH), 6.80 (dd, J = 2.1 Hz, J =
8.3 Hz, 1H, H-7''), 6.84 (d, J = 2.1 Hz, 1H, H-5''), 6.85 (d, J = 8.3 Hz, 1H, H-8''), 7.05 (d, J = 8.8 Hz,
2H, H-3',5'), 7.68 (d, J = 8.8 Hz, 2H, H-2',6'), 10.01 (s, 1H, NH). 13
C NMR (DMSO-d6) δ 169.8,
160.0, 156.4, 147.9, 143.5, 142.8, 134.0, 133.5, 129.1, 126.2, 119.9, 117.4, 115.8, 114.1, 64.1, 64.0,
55.3, 38.4, 36.8. EIMS m/z 394 [M]+
(100), 379 (6), 365 (12), 363 (15), 351 (24), 335 (10), 260 (21),
233 (14), 202 (13), 197 (19), 161 (13), 136 (22), 134 (16), 108 (13), 77 (12), 63 (8). Anal. Calcd for
C21H18N2O4S: C, 63.94; H, 4.60; N, 7.10. Found: C, 63.86; H, 4.56; N, 7.16.
4.1.3.7. 6,7-Dihydro-7-(7-methoxy-1,3-benzodioxol-5-yl)-3-(4-methoxyphenyl)-isothiazolo[4,5-
b]pyridin-5(4H)-one (8bk). Yellowish solid, mp 140–142 °C. 1
H NMR (DMSO-d6, 500 MHz): δ
2.78 (dd, J = 6.1 Hz, J = 15.5 Hz, 1H, CH2), 2.95 (dd, J = 10.1 Hz, J = 15.5 Hz, 1H, CH2), 3.82 (s,
6H, OCH3-4',7''), 4.61 (dd, J = 6.1 Hz, J = 10.1 Hz, 1H, CH), 5.99 (s, 2H, OCH2O), 6.61 (d, J = 1.5
Hz, 1H, H-4''), 6.70 (d, J = 1.5 Hz, 1H, H-6''), 7.05 (d, J = 8.8 Hz, 2H, H-3',5'), 7.68 (d, J = 8.8 Hz,
2H, H-2',6'), 10.02 (s, 1H, NH). EIMS m/z 410 [M]+
(100), 409 (23), 395 (7), 379 (26), 367 (18),
352 (16), 337 (14), 259 (21), 246 (23), 205 (36), 163 (10), 147 (10), 134 (19), 119 (10), 90 (10), 77
(19), 63 (13). Anal. Calcd for C21H18N2O5S: C, 61.45; H, 4.42; N, 6.83. Found: C, 61.39; H, 4.40; N,
6.79.
4.1.3.8. 7-(6,7-Dimethoxy-1,3-benzodioxol-5-yl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-
b]pyridin-5(4H)-one (8bl). Yellowish solid, mp 187–188 °C. 1
H NMR (DMSO-d6, 500 MHz): δ
2.82 (m, 2H, CH2), 3.76 (s, 3H, OCH3-6''), 3.82 (s, 3H, OCH3-4'), 3.96 (s, 3H, OCH3-7''), 4.80 (t, J =
7.3 Hz, 1H, CH), 5.98 (s, 2H, OCH2O), 6.46 (s, 1H, H-4''), 7.05 (d, J = 8.8 Hz, 2H, H-3',5'), 7.67 (d,
J = 8.8 Hz, 2H, H-2',6'), 10.00 (s, 1H, NH). EIMS m/z 440 [M]+
(100), 425 (37), 409 (33), 367 (12),
352 (12), 276 (15), 275 (40), 259 (34), 247 (22), 220 (34), 182 (62), 167 (11), 134 (19), 77 (11).
Anal. Calcd for C22H20N2O6S: C, 59.99; H, 4.58; N, 6.36. Found: C, 60.10; H, 4.60; N, 6.27.
4.1.3.9. 7-(4,7-Dimethoxy-1,3-benzodioxol-5-yl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-
b]pyridin-5(4H)-one (8bm). Yellow solid, mp 191–192 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.86
(m, 2H, CH2), 3.73 (s, 3H, OCH3-4''), 3.82 (s, 3H, OCH3-4'), 3.88 (s, 3H, OCH3-7''), 4.78 (t, J = 7.1
Hz, 1H, CH), 6.03 (s, 2H, OCH2O), 6.48 (s, 1H, H-6''), 7.04 (d, J = 8.8 Hz, 2H, H-3',5'), 7.67 (d, J =
8.8 Hz, 2H, H-2',6'), 9.98 (s, 1H, NH). 13
C NMR (DMSO-d6) δ 169.9, 160.0, 156.1, 147.1, 138.8,
138.5, 136.1, 135.4, 133.5, 129.1, 126.2, 125.9, 114.1, 106.6, 101.9, 60.0, 56.8, 55.3, 37.0, 32.0.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-19-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
18
EIMS m/z 440 [M]+
(27), 439 (18), 409 (10), 259 (46), 220 (61), 182 (100), 167 (33), 161 (17), 149
(58), 135 (42), 134 (79), 133 (55), 121 (31), 109 (38), 108 (36), 103 (38), 90 (74), 77 (96), 63 (81).
Anal. Calcd for C22H20N2O6S: C, 59.99; H, 4.58; N, 6.36. Found: C, 60.08; H, 4.61; N, 6.29.
4.1.3.10. 7-(3,4-Dihydroxy-2,5-dimethoxyphenyl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-
b]pyridin-5(4H)-one (8bn). White solid, mp 203–205 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.74
(dd, J = 6.4 Hz, J = 15.6 Hz, 1H, CH2), 2.93 (dd, J = 10.1 Hz, J = 15.6 Hz, 1H, CH2), 3.66 (s, 3H,
OCH3-2''), 3.71 (s, 3H, OCH3-5''), 3.82 (s, 3H, OCH3-4'), 4.75 (dd, J = 6.4 Hz, J = 10.1 Hz, 1H,
CH), 6.34 (s, 1H, H-6''), 7.05 (d, J = 8.6 Hz, 2H, H-3',5'), 7.67 (d, J = 8.6 Hz, 2H, H-2',6'), 8.63
(br.s, 1H, OH-3''), 8.71 (br.s, 1H, OH-4''), 9.98 (s, 1H, NH). EIMS m/z 428 [M]+
(100), 413 (22),
397 (29), 355 (15), 263 (33), 259 (88), 214 (32), 170 (55), 155 (14), 149 (15), 134 (29), 109 (11), 77
(11). Anal. Calcd for C21H20N2O6S: C, 58.87; H, 4.70; N, 6.54. Found: C, 58.81; H, 4.67; N, 6.62.
4.1.3.11. 7-(3-Chlorophenyl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-one
(8bo). Yellowish solid, mp 189–191 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.92 (d, J = 7.7 Hz, 2H,
CH2), 3.82 (s, 3H, OCH3-4'), 4.78 (t, J = 7.7 Hz, 1H, CH), 7.05 (d, J = 8.8 Hz, 2H, H-3',5'), 7.32 (dt,
J = 1.5 Hz, J = 7.5 Hz, 1H, H-6''), 7.38 (dt, J = 1.5 Hz, J = 7.5 Hz, 1H, H-4''), 7.42 (d, J = 7.5 Hz,
1H, H-5''), 7.43 (t, J = 1.5 Hz, 1H, H-2''), 7.69 (d, J = 8.8 Hz, 2H, H-2',6'), 10.06 (s, 1H, NH). 13
C
NMR (DMSO-d6) δ 169.6, 160.0, 156.5, 146.6, 143.5, 133.9, 133.5, 130.9, 129.2, 127.6, 127.3,
126.1, 126.0, 114.1, 55.3, 38.0, 37.1. EIMS m/z 372 [M+2]+
(36), 370 [M]+
(100), 355 (6), 341 (10),
327 (14), 202 (12), 170 (10), 168 (50), 146 (11), 134 (51), 133 (59), 103 (54), 102 (47), 101 (31), 90
(77), 89 (60), 77 (56), 75 (79), 63 (93). Anal. Calcd for C19H15ClN2O2S: C, 61.54; H, 4.08; N, 7.55.
Found: C, 61.67; H, 4.11; N, 7.44.
4.1.3.12. 7-(4-Chlorophenyl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-one
(8bp). White solid, mp 194–196 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.90 (d, J = 7.5 Hz, 2H,
CH2), 3.82 (s, 3H, OCH3-4'), 4.77 (t, J = 7.5 Hz, 1H, CH), 7.05 (d, J = 8.8 Hz, 2H, H-3',5'), 7.38 (d,
J = 8.5 Hz, 2H, H-2'',6''), 7.45 (d, J = 8.5 Hz, 2H, H-3'',5''), 7.68 (d, J = 8.8 Hz, 2H, H-2',6'), 10.05
(s, 1H, NH). EIMS m/z 372 [M+2]+
(34), 370 [M]+
(100), 357 (2), 355 (5), 341 (9), 335 (11), 327
(19), 202 (22), 170 (17), 168 (52), 146 (12), 137 (14), 134 (40), 133 (48), 131 (11), 125 (13), 115
(10), 103 (38), 102 (33), 101 (24), 90 (50), 89 (43), 77 (40), 63 (61). Anal. Calcd for
C19H15ClN2O2S: C, 61.54; H, 4.08; N, 7.55. Found: C, 61.64; H, 4.10; N, 7.47.
4.1.3.13. 7-(3-Fluorophenyl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-one
(8bq). Yellowish solid, mp 175–177 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.90 (dd, J = 6.7 Hz, J =](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-20-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
19
15.6 Hz, 1H, CH2), 2.94 (dd, J = 8.4 Hz, J = 15.6 Hz, 1H, CH2), 3.82 (s, 3H, OCH3-4'), 4.79 (dd, J =
6.7 Hz, J = 8.4 Hz, 1H, CH), 7.05 (d, J = 8.8 Hz, 2H, H-3',5'), 7.15 (dt, J = 2.5 Hz, J = 9.8 Hz, 1H,
H-6''), 7.21 (m, 2H, H-2'',4''), 7.44 (dt, J = 8.1 Hz, J = 11.2 Hz, 1H, H-5''), 7.69 (d, J = 8.8 Hz, 2H,
H-2',6'), 10.07 (s, 1H, NH). 13
C NMR (DMSO-d6) δ 169.6, 163.4, 161.4, 160.0, 156.5, 146.7, 143.8,
143.7, 133.8, 131.1, 131.0, 129.2, 126.1, 123.4, 114.6, 114.4, 114.3, 114.1, 55.3, 38.0, 37.2. EIMS
m/z 354 [M]+
(96), 339 (4), 325 (7), 311 (21), 259 (18), 233 (15), 220 (34), 177 (28), 165 (28), 161
(26), 156 (14), 152 (100), 149 (13), 134 (88), 133 (57), 121 (38), 108 (49), 107 (41), 101 (42), 95
(21), 90 (38), 77 (33), 75 (30), 63 (36). Anal. Calcd for C19H15FN2O2S: C, 64.39; H, 4.27; N, 7.90.
Found: C, 64.47; H, 4.31; N, 7.80.
4.1.3.14. 7-(4-Fluorophenyl)-6,7-dihydro-3-(4-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-one
(8br). Yellowish solid, mp 206–208 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.87 (dd, J = 6.6 Hz, J =
12.1 Hz, 1H, CH2), 2.91 (dd, J = 8.7 Hz, J = 12.1 Hz, 1H, CH2), 3.82 (s, 3H, OCH3-4'), 4.76 (dd, J =
6.6 Hz, J = 8.7 Hz, 1H, CH), 7.05 (d, J = 8.8 Hz, 2H, H-3',5'), 7.22 (t, J = 8.8 Hz, 2H, H-3'',5''), 7.40
(dd, J = 5.4 Hz, J = 8.8 Hz, 2H, H-2'',6''), 7.68 (d, J = 8.8 Hz, 2H, H-2',6'), 10.04 (s, 1H, NH). 13
C
NMR (DMSO-d6) δ 169.7, 162.5, 161.0, 160.0, 156.5, 147.4, 137.2, 133.7, 129.4, 129.3, 129.2,
126.2, 115.8, 115.7, 114.1, 55.3, 38.3, 36.8. EIMS m/z 354 [M]+
(74), 339 (6), 325 (10), 311 (35),
233 (16), 220 (43), 177 (24), 165 (21), 161 (26), 156 (21), 152 (100), 149 (25), 134 (71), 133 (50),
121 (39), 108 (44), 107 (37), 101 (34), 95 (19), 90 (34), 77 (22), 75 (31), 63 (38). Anal. Calcd for
C19H15FN2O2S: C, 64.39; H, 4.27; N, 7.90. Found: C, 64.45; H, 4.30; N, 7.83.
4.1.3.15. 7-(3-Chlorophenyl)-6,7-dihydro-3-(3,4-dimethoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-
one (8co). Yellowish solid, mp 205–206 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.80 (dd, J = 6.1 Hz,
J = 15.6 Hz, 1H, CH2), 2.97 (dd, J = 10.2 Hz, J = 15.6 Hz, 1H, CH2), 3.74 (s, 6H, OCH3-3',4'), 4.65
(dd, J = 6.1 Hz, J = 10.2 Hz, 1H, CH), 6.85 (d, J = 8.2 Hz, 1H, H-5'), 6.94 (d, J = 8.2 Hz, 1H, H-6'),
7.02 (s, 1H, H-2'), 7.53 (m, 2H, H-4'',6''), 7.68 (m, 1H, H-5''), 7.72 (s, 1H, H-2''), 10.29 (s, 1H, NH);
13
C NMR (DMSO-d6, 150 MHz): δ 37.2, 38.2, 55.4, 55.5, 111.0, 111.9, 119.0, 126.3, 127.3, 128.9,
130.5, 133.0, 133.2, 133.8, 135.2, 148.1, 148.9, 149.0, 154.7, 170.0; EIMS m/z 402 [M+2]+
(33),
400 [M]+
(100), 385 (3), 371 (6), 357 (12), 341 (8), 327 (7), 202 (8), 170 (11), 168 (43), 146 (9), 134
(35), 133 (41), 103 (50), 102 (43), 101 (28), 90 (67), 89 (56), 77 (52), 75 (54), 63 (89). Anal. Calcd
for C20H17ClN2O3S: C, 59.92; H, 4.27; N, 6.99. Found: C, 60.03; H, 4.23; N, 6.87.
4.1.3.16. 3-(4-Chlorophenyl)-7-(7-methoxy-1,3-benzodioxol-5-yl)-6,7-dihydro-isothiazolo[4,5-
b]pyridin-5(4H)-one (8dk). White solid, mp 202–204 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.80
(dd, J = 6.2 Hz, J = 15.5 Hz, 1H, CH2), 2.94 (dd, J = 10.0 Hz, J = 15.5 Hz, 1H, CH2), 3.82 (s, 3H,](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-21-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
20
OCH3-7''), 4.64 (dd, J = 6.2 Hz, J = 10.0 Hz, 1H, CH), 5.99 (s, 2H, OCH2O), 6.61 (d, J = 1.3 Hz,
1H, H-4''), 6.69 (d, J = 1.3 Hz, 1H, H-6''), 7.56 (d, J = 8.5 Hz, 2H, H-2',6'), 7.74 (d, J = 8.5 Hz, 2H,
H-3',5'), 10.11 (s, 1H, NH). EIMS m/z 416 [M+2]+
(2), 414 [M]+
(6), 178 (51), 165 (16), 163 (23),
147 (11), 139 (20), 138 (37), 137 (44), 121 (25), 111 (40), 102 (68), 95 (18), 94 (10), 93 (19), 77
(92), 75 (100), 63 (77). Anal. Calcd for C20H15ClN2O4S: C, 57.90; H, 3.64; N, 6.75. Found: C,
57.96; H, 3.66; N, 6.84.
4.1.3.17. 3-(4-Chlorophenyl)-7-(4,7-dimethoxy-1,3-benzodioxol-5-yl)-6,7-dihydro-isothiazolo
[4,5-b]pyridin-5(4H)-one (8dm). White solid, mp 205–207 °C. 1
H NMR (DMSO-d6, 500 MHz): δ
2.86 (m, 2H, CH2), 3.73 (s, 3H, OCH3-4''), 3.88 (s, 3H, OCH3-7''), 4.80 (t, J = 7.3 Hz, 1H, CH), 6.03
(s, 2H, OCH2O), 6.48 (s, 1H, H-6''), 7.55 (d, J = 8.5 Hz, 2H, H-2',6'), 7.73 (d, J = 8.5 Hz, 2H, H-
3',5'), 10.11 (s, 1H, NH). EIMS m/z 446 [M+2]+
(18), 444 [M]+
(54), 413 (23), 371 (15), 265 (13),
263 (42), 247 (21), 223 (25), 222 (29), 182 (100), 178 (20), 167 (37), 163 (21), 149 (15), 148 (12),
147 (19), 135 (35), 123 (26), 122 (27), 121 (22), 111 (58), 102 (74), 95 (33), 94 (27), 93 (42), 77
(69). Anal. Calcd for C21H17ClN2O5S: C, 56.69; H, 3.85; N, 6.30. Found: C, 5.73; H, 3.89; N, 6.20.
4.1.3.18. 3-(3-Fluorophenyl)-6,7-dihydro-7-(4-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-one
(8eg). White solid, mp 169–170 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.83 (dd, J = 6.4 Hz, J =
15.6 Hz, 1H, CH2), 2.90 (dd, J = 9.3 Hz, J = 15.6 Hz, 1H, CH2), 3.75 (s, 3H, OCH3-4''), 4.69 (dd, J
= 6.4 Hz, J = 9.3 Hz, 1H, CH), 6.94 (d, J = 8.7 Hz, 2H, H-3'',5''), 7.28 (d, J = 8.7 Hz, 2H, H-2'',6''),
7.31 (m, 1H, HAr), 7.50 (m, 1H, HAr), 7.56 (m, 2H, HAr), 10.20 (s, 1H, NH). EIMS m/z 354 [M]+
(15), 325 (6), 323 (14), 311 (18), 297 (11), 247 (15), 202 (20), 177 (16), 164 (15), 149 (69), 134
(28), 133 (27), 122 (47), 121 (100), 108 (25), 103 (16), 102 (2), 101 (8), 95 (61), 94 (45), 91 (42), 89
(44), 77 (80), 75 (68), 63 (76). Anal. Calcd for C19H15FN2O2S: C, 64.39; H, 4.27; N, 7.90. Found: C,
64.47; H, 4.31; N, 7.82.
4.1.3.19. 3-(3-Fluorophenyl)-6,7-dihydro-7-(3-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-one
(8eh). White solid, mp 153–155 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.88 (dd, J = 6.5 Hz, J =
15.6 Hz, 1H, CH2), 2.93 (dd, J = 8.8 Hz, J = 15.6 Hz, 1H, CH2), 3.75 (s, 3H, OCH3-3''), 4.73 (dd, J
= 6.5 Hz, J = 8.8 Hz, 1H, CH), 6.90 (m, 3H, HAr), 7.31 (m, 2H, HAr), 7.53 (m, 3H, HAr), 10.21 (s,
1H, NH). EIMS m/z 354 [M]+
(44), 339 (2), 325 (10), 323 (12), 311 (17), 281 (20), 247 (27), 202
(19), 177 (15), 164 (17), 156 (13), 147 (20), 134 (41), 133 (27), 122 (63), 121 (100), 108 (21), 103
(30), 102 (37), 101 (15), 95 (78), 94 (50), 91 (50), 89 (52), 77 (86), 75 (77), 63 (75). Anal. Calcd for
C19H15FN2O2S: C, 64.39; H, 4.27; N, 7.90. Found: C, 64.42; H, 4.30; N, 7.85.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-22-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
21
4.1.3.20. 7-(1,3-Benzodioxol-5-yl)-3-(3-fluorophenyl)-6,7-dihydro-isothiazolo[4,5-b]pyridin-5(4H)-
one (8ei). Yellow solid, mp 197–199 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.82 (dd, J = 6.3 Hz, J
= 15.5 Hz, 1H, CH2), 2.90 (dd, J = 9.3 Hz, J = 15.5 Hz, 1H, CH2), 4.67 (dd, J = 6.3 Hz, J = 9.3 Hz,
1H, CH), 6.01 (s, 1H, OCH2O), 6.02 (s, 1H, OCH2O), 6.81 (d, J = 8.0 Hz, 1H, H-6''), 6.90 (d, J =
8.0 Hz, 1H, H-7''), 6.95 (s, 1H, H-4''), 7.31 (t, J = 7.8 Hz, 1H, HAr), 7.50 (m, 1H, HAr), 7.55 (m, 2H,
HAr), 10.16 (s, 1H, NH). EIMS m/z 368 [M]+
(6), 247 (6), 217 (7), 178 (24), 177 (15), 161 (13), 147
(50), 134 (28), 122 (64), 121 (82), 120 (71), 102 (24), 95 (74), 94 (61), 89 (100), 77 (34), 76 (41), 75
(68), 63 (99). Anal. Calcd for C19H13FN2O3S: C, 61.95; H, 3.56; N, 7.60. Found: C, 61.90; H, 3.52;
N, 7.67.
4.1.3.21. 3-(3-Fluorophenyl)-6,7-dihydro-7-(7-methoxy-1,3-benzodioxol-5-yl)-isothiazolo[4,5-
b]pyridin-5(4H)-one (8ek). Yellowish solid, mp 180–182 °C. 1
H NMR (DMSO-d6, 500 MHz): δ
2.80 (dd, J = 6.2 Hz, J = 15.6 Hz, 1H, CH2), 2.95 (dd, J = 10.0 Hz, J = 15.6 Hz, 1H, CH2), 3.82 (s,
3H, OCH3-7''), 4.65 (dd, J = 6.2 Hz, J = 10.0 Hz, 1H, CH), 6.00 (s, 2H, OCH2O), 6.61 (d, J = 1.5
Hz, 1H, H-4''), 6.70 (d, J = 1.5 Hz, 1H, H-6''), 7.31 (m, 1H, HAr), 7.50 (m, 1H, HAr), 7.55 (m, 2H,
HAr), 10.19 (s, 1H, NH). EIMS m/z 398 [M]+
(100), 369 (10), 367 (26), 340 (17), 339 (15), 325 (17),
247 (22), 177 (17), 163 (25), 148 (17), 147 (17), 146 (17), 135 (21), 134 (21), 133 (21), 122 (55),
121 (53), 107 (24), 95 (62), 94 (25), 93 (15), 77 (53). Anal. Calcd for C20H15FN2O4S: C, 60.29; H,
3.79; N, 7.03. Found: C, 60.33; H, 3.81; N, 6.97.
4.1.3.22. 7-(4,7-Dimethoxy-1,3-benzodioxol-5-yl)-3-(3-fluorophenyl)-6,7-dihydro-isothiazolo[4,5-
b]pyridin-5(4H)-one (8em). Brown solid, mp 147–149 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.87
(m, 2H, CH2), 3.73 (s, 3H, OCH3-4''), 3.88 (s, 3H, OCH3-7''), 4.81 (t, J = 7.3 Hz, 1H, CH), 6.03 (s,
2H, OCH2O), 6.48 (s, 1H, H-6''), 7.30 (m, 1H, HAr), 7.49 (m, 1H, HAr), 7.54 (m, 2H, HAr), 10.18 (s,
1H, NH). EIMS m/z 428 [M]+
(86), 427 (40), 397 (32), 355 (19), 247 (52), 229 (11), 214 (19), 182
(79), 177 (13), 167 (29), 163 (17), 149 (18), 148 (19), 147 (19), 135 (37), 123 (32), 122 (91), 121
(55), 109 (28), 95 (100), 94 (48), 93 (39), 77 (59). Anal. Calcd for C21H17FN2O5S: C, 58.87; H, 4.00;
N, 6.54. Found: C, 58.95; H, 4.03; N, 6.41.
4.1.3.23. 7-(3-Chlorophenyl)-3-(3-fluorophenyl)-6,7-dihydro-isothiazolo[4,5-b]pyridin-5(4H)-one
(8eo). Yellowish solid, mp 148–150 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.93 (d, J = 7.4 Hz, 2H,
CH2), 4.82 (t, J = 7.4 Hz, 1H, CH), 7.32 (m, 2H, HAr), 7.41 (m, 3H, HAr), 7.54 (m, 3H, HAr), 10.25
(s, 1H, NH). EIMS m/z 360 [M+2] (0.6), 358 [M]+
(2), 202 (7), 170 (6), 168 (15), 133 (14), 122
(19), 121 (29), 102 (21), 101 (19), 95 (38), 94 (32), 89 (33), 75 (100), 71 (41), 70 (42), 69 (42).
Anal. Calcd for C18H12ClFN2OS: C, 60.25; H, 3.37; N, 7.81. Found: C, 60.33; H, 3.41; N, 7.74.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-23-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
22
4.1.3.24. 7-(4-Chlorophenyl)-3-(3-fluorophenyl)-6,7-dihydro-isothiazolo[4,5-b]pyridin-5(4H)-one
(8ep). Yellowish solid, mp 201–202 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.91 (d, J = 7.6 Hz, 2H,
CH2), 4.80 (t, J = 7.6 Hz, 1H, CH), 7.31 (m, 1H, HAr), 7.37 (d, J = 8.5 Hz, 2H, H-2'',6''), 7.46 (d, J =
8.5 Hz, 2H, H-3'',5''), 7.50 (m, 1H, HAr), 7.54 (m, 2H, HAr), 10.24 (s, 1H, NH). EIMS m/z 360 [M+2]
(6), 358 [M]+
(18), 323 (25), 315 (25), 281 (22), 247 (31), 221 (15), 202 (55), 174 (33), 170 (37),
168 (100), 147 (42), 140 (51), 133 (67), 122 (64), 121 (64), 102 (56), 101 (56), 95 (61), 94 (46), 89
(77), 75 (96), 71 (54), 70 (53), 69 (49). Anal. Calcd for C18H12ClFN2OS: C, 60.25; H, 3.37; N, 7.81.
Found: C, 60.34; H, 3.39; N, 7.72.
4.1.3.25. 3,7-bis(3-Fluorophenyl)-6,7-dihydro-isothiazolo[4,5-b]pyridin-5(4H)-one (8eq).
Yellowish solid, mp 165–167 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.93 (m, 2H, CH2), 4.82 (t, J =
7.6 Hz, 1H, CH), 7.18 (m, 3H, HAr), 7.32 (m, 1H, HAr), 7.44 (m, 1H, HAr), 7.51 (m, 1H, HAr), 7.56
(m, 2H, HAr), 10.25 (s, 1H, NH); EIMS m/z 342 [M]+
(34), 313 (15), 299 (42), 247 (29), 221 (15),
220 (26), 165 (21), 152 (100), 148 (18), 134 (20), 133 (47), 122 (58), 121 (79), 120 (34), 108 (51),
107 (61), 101 (61), 96 (44), 95 (92), 94 (48), 75 (97), 71 (75), 70 (76), 69 (69). Anal. Calcd for
C18H12F2N2OS: C, 63.15; H, 3.53; N, 8.18. Found: C, 53.26; H, 3.56; N, 8.07.
4.1.3.26. 3-(3-Fluorophenyl)-7-(4-fluorophenyl)-6,7-dihydro-isothiazolo[4,5-b]pyridin-5(4H)-one
(8er). White solid, mp 179-181 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.90 (m, 2H, CH2), 4.79 (t, J
= 7.4 Hz, 1H, CH), 7.22 (t, J = 8.8 Hz, 2H, H-3'',5''), 7.31 (m, 1H, HAr), 7.40 (m, 2H, HAr), 7.51 (m,
1H, HAr), 7.56 (m, 2H, HAr), 10.23 (s, 1H, NH). EIMS m/z 342 [M]+
(2), 247 (10), 220 (18), 179
(12), 178 (15), 165 (18), 161 (16), 152 (100), 148 (15), 139 (15), 133 (44), 122 (63), 121 (98), 120
(40), 109 (33), 108 (55), 107 (65), 101 (56), 96 (41), 95 (82), 94 (56), 75 (96), 71 (51), 70 (6), 69
(66). Anal. Calcd for C18H12F2N2OS: C, 63.15; H, 3.53; N, 8.18. Found: C, 63.19; H, 3.54; N, 8.12.
4.1.3.27. 3-(4-Fluorophenyl)-6,7-dihydro-7-(4-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-one
(8fg). Yellowish solid, mp 216–217 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.83 (dd, J = 6.4 Hz, J =
15.6 Hz, 1H, CH2), 2.89 (dd, J = 9.3 Hz, J = 15.6 Hz, 1H, CH2), 3.75 (s, 3H, OCH3-4''), 4.67 (dd, J
= 6.4 Hz, J = 9.3 Hz, 1H, CH), 6.94 (d, J = 8.7 Hz, 2H, H-3'',5''), 7.28 (d, J = 8.7 Hz, 2H, H-2'',6''),
7.32 (t, J = 8.8 Hz, 2H, H-3',5'), 7.76 (dd, J = 5.5 Hz, J = 8.8 Hz, 2H, H-2',6'), 10.09 (s, 1H, NH).
EIMS m/z 354 [M]+
(100), 339 (2), 325 (17), 323 (35), 311 (53), 297 (27), 281 (15), 268 (19), 247
(34), 232 (23), 218 (10), 202 (24), 177 (11), 164 (10), 149 (40), 121 (51), 119 (10), 95 (25), 91 (15),
77 (24), 63 (20). Anal. Calcd for C19H15FN2O2S: C, 64.39; H, 4.27; N, 7.90. Found: C, 64.43; H,
4.30; N, 7.82.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-24-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
23
4.1.3.28. 3-(4-Fluorophenyl)-6,7-dihydro-7-(3-methoxyphenyl)-isothiazolo[4,5-b]pyridin-5(4H)-one
(8fh). White solid, mp 141–143 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.87 (dd, J = 6.6 Hz, J =
15.6 Hz, 1H, CH2), 2.92 (dd, J = 8.8 Hz, J = 15.6 Hz, 1H, CH2), 3.75 (s, 3H, OCH3-3''), 4.71 (dd, J
= 6.6 Hz, J = 8.8 Hz, 1H, CH), 6.90 (m, 3H, HAr), 7.31 (m, 3H, HAr), 7.76 (dd, J = 5.5 Hz, J = 8.8
Hz, 2H, H-2',6'), 10.10 (s, 1H, NH). EIMS m/z 354 [M]+
(41), 325 (5), 323 (5), 311 (12), 281 (1),
247 (17), 202 (18), 177 (13), 164 (14), 147 (16), 134 (35), 133 (18), 122 (59), 121 (100), 108 (14),
103 (22), 102 (26), 95 (77), 94 (48), 91 (44), 89 (42), 77 (72), 75 (67), 63 (70). Anal. Calcd for
C19H15FN2O2S: C, 64.39; H, 4.27; N, 7.90. Found: C, 64.40; H, 4.28; N, 7.87.
4.1.3.29. 7-(1,3-Benzodioxol-5-yl)-3-(4-fluorophenyl)-6,7-dihydro-isothiazolo[4,5-b]pyridin-5(4H)-
one (8fi). Yellow solid, mp 195–197 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.81 (dd, J = 6.3 Hz, J =
15.5 Hz, 1H, CH2), 2.91 (dd, J = 9.5 Hz, J = 15.5 Hz, 1H, CH2), 4.66 (dd, J = 6.3Hz, J = 9.5 Hz, 1H,
CH), 6.02 (s, 2H, OCH2O), 6.81 (dd, J = 1.8 Hz, J = 8.0 Hz, 1H, H-6''), 6.91 (d, J = 8.0 Hz, 1H, H-
7''), 6.96 (d, J = 1.8 Hz, 1H, H-4''), 7.33 (t, J = 8.9 Hz, 2H, H-3',5'), 7.76 (dd, J = 5.5 Hz, J = 8.9 Hz,
2H, H-2',6'), 10.14 (s, 1H, NH). EIMS m/z 368 [M]+
(100), 339 (15), 325 (35), 247 (18), 246 (16),
178 (27), 177 (15), 161 (12), 148 (23), 147 (39), 146 (31), 134 (22), 133 (12), 122 (58), 121 (81),
120 (57), 102 (18), 95 (66), 94 (53), 89 (80). Anal. Calcd for C19H13FN2O3S: C, 61.95; H, 3.56; N,
7.60. Found: C, 62.00; H, 3.58; N, 7.54.
4.1.3.30. 3-(4-Fluorophenyl)-6,7-dihydro-7-(7-methoxy-1,3-benzodioxol-5-yl)-isothiazolo[4,5-
b]pyridin-5(4H)-one (8fk). Yellowish solid, mp 200–201 °C. 1
H NMR (DMSO-d6, 500 MHz): δ
2.80 (dd, J = 6.2 Hz, J = 15.5 Hz, 1H, CH2), 2.95 (dd, J = 10.0 Hz, J = 15.5 Hz, 1H, CH2), 3.82 (s,
3H, OCH3-7''), 4.64 (dd, J = 6.2 Hz, J = 10.0 Hz, 1H, CH), 6.00 (s, 2H, OCH2O), 6.61 (d, J = 1.5
Hz, 1H, H-4''), 6.70 (d, J = 1.5 Hz, 1H, H-6''), 7.33 (t, J = 8.9 Hz, 2H, H-3',5'), 7.76 (dd, J = 5.5 Hz,
J = 8.9 Hz, 2H, H-2',6'), 10.14 (s, 1H, NH). EIMS m/z 398 [M]+
(100), 369 (7), 367 (17), 355 (9),
340 (9), 339 (8), 325 (8), 122 (6), 121 (6), 95 (6). Anal. Calcd for C20H15FN2O4S: C, 60.29; H, 3.79;
N, 7.03. Found: C, 60.35; H, 3.82; N, 6.96.
4.1.3.31. 7-(4,7-Dimethoxy-1,3-benzodioxol-5-yl)-3-(4-fluorophenyl)-6,7-dihydro-isothiazolo[4,5-
b]pyridin-5(4H)-one (8fm). White solid, mp 166–168 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.86
(m, 2H, CH2), 3.73 (s, 3H, OCH3-4''), 3.88 (s, 3H, OCH3-7''), 4.80 (t, J = 7.6 Hz, 1H, CH), 6.03 (s,
2H, OCH2O), 6.48 (s, 1H, H-6''), 7.32 (t, J = 8.8 Hz, 2H, H-3',5'), 7.75 (dd, J = 5.5 Hz, J = 8.8 Hz,
2H, H-2',6'), 10.10 (s, 1H, NH). EIMS m/z 428 [M]+
(38), 427 (16), 397 (15), 355 (10), 247 (41),
214 (24), 182 (69), 177 (15), 167 (25), 163 (16), 149 (17), 148 (17), 147 (16), 135 (32), 123 (30),](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-25-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
24
122 (100), 121 (85), 109 (25), 95 (98), 94 (61), 93 (41), 77 (45), 63 (36). Anal. Calcd for
C21H17FN2O5S: C, 58.87; H, 4.00; N, 6.54. Found: C, 58.93; H, 4.03; N, 6.47.
4.1.3.32. 7-(3-Chlorophenyl)-3-(4-fluorophenyl)-6,7-dihydro-isothiazolo[4,5-b]pyridin-5(4H)-one
(8fo). Yellow solid, mp 167–169 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.93 (d, J = 7.4 Hz, 2H,
CH2), 4.80 (t, J = 7.4 Hz, 1H, CH), 7.33 (m, 3H, HAr), 7.41 (m, 3H, HAr), 7.77 (dd, J = 5.5 Hz, J =
8.7 Hz, 2H, H-2',6'), 10.16 (s, 1H, NH). EIMS m/z 360 [M+2] (22), 358 [M]+
(65), 323 (10), 315
(18), 281 (19), 247 (26), 202 (26), 174 (17), 170 (16), 168 (46), 147 (18), 140 (22), 133 (33), 122
(41), 121 (57), 102 (35), 101 (30), 95 (56), 94 (46), 89 (52), 75 (100), 71 (10), 70 (48), 69 (45).
Anal. Calcd for C18H12ClFN2OS: C, 60.25; H, 3.37; N, 7.81. Found: C, 60.31; H, 3.40; N, 7.75.
4.1.3.33. 3,7-bis(4-Fluorophenyl)-6,7-dihydro-isothiazolo[4,5-b]pyridin-5(4H)-one (8fr). White
solid, mp 254–256 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.90 (m, 2H, CH2), 4.78 (t, J = 7.1 Hz,
1H, CH), 7.22 (t, J = 8.8 Hz, 2H, H-3'',5''), 7.32 (t, J = 8.8 Hz, 2H, H-3',5'), 7.40 (dd, J = 5.5 Hz, J =
8.8 Hz, 2H, H-2'',6''), 7.76 (dd, J = 5.5 Hz, J = 8.8 Hz, 2H, H-2',6'), 10.14 (s, 1H, NH); EIMS m/z
342 [M]+
(41), 299 (36), 247 (5), 220 (19), 165 (13), 152 (89), 148 (15), 134 (11), 133 (23), 122
(43), 121 (100), 120 (22), 108 (27), 107 (35), 101 (38), 96 (34), 95 (67), 94 (57), 75 (80), 71 (25), 70
(39), 69 (38). Anal. Calcd for C18H12F2N2OS: C, 63.15; H, 3.53; N, 8.18. Found: C, 63.18; H, 3.56;
N, 8.11.
4.1.3.34. 6,7-Dihydro-3-(4-methoxyphenyl)-7-(3-thienyl)-isothiazolo[4,5-b]pyridin-5(4H)-one (9b).
Brown solid, mp 153–155 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.87 (dd, J = 6.3 Hz, J = 15.6 Hz,
1H, CH2), 2.94 (dd, J = 8.6 Hz, J = 15.6 Hz, 1H, CH2), 3.82 (s, 3H, OCH3-4'), 4.81 (dd, J = 6.3 Hz,
J = 8.6 Hz, 1H, CH), 7.04 (d, J = 8.8 Hz, 2H, H-3',5'), 7.16 (dd, J = 1.3 Hz, J = 5.0 Hz, 1H, H-4''),
7.36 (d, J = 2.9 Hz, 1H, H-2''), 7.58 (dd, J = 2.9 Hz, J = 5.0 Hz, 1H, H-5''), 7.67 (d, J = 8.8 Hz, 2H,
H-2',6'), 10.00 (s, 1H, NH). 13
C NMR (DMSO-d6) δ 169.8, 160.0, 156.4, 147.4, 141.3, 133.3, 129.2,
127.4, 126.9, 126.2, 122.0, 114.1, 55.3, 37.8, 33.2. EIMS m/z 342 [M]+
(100), 341 (34), 299 (43),
233 (12), 209 (16), 208 (44), 149 (28), 140 (54), 134 (33), 121 (15), 109 (29), 108 (16), 96 (35), 90
(34), 77 (22), 63 (37). Anal. Calcd for C17H14N2O2S2: C, 59.63; H, 4.12; N, 8.18. Found: C, 59.71;
H, 4.16; N, 8.04.
4.1.3.35. 3-(4-Chlorophenyl)-6,7-dihydro-7-(3-thienyl)-isothiazolo[4,5-b]pyridin-5(4H)-one (9d).
Yellowish solid, mp 210–212 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.88 (dd, J = 6.4 Hz, J = 15.6
Hz, 1H, CH2), 2.94 (dd, J = 8.4 Hz, J = 15.6 Hz, 1H, CH2), 4.84 (dd, J = 6.4 Hz, J = 8.4 Hz, 1H,
CH), 7.15 (dd, J = 1.2 Hz, J = 5.0 Hz, 1H, H-4''), 7.36 (d, J = 2.9 Hz, 1H, H-2''), 7.55 (d, J = 8.5 Hz,](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-26-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
25
2H, H-3',5'), 7.58 (dd, J = 2.9 Hz, J = 5.0 Hz, 1H, H-5''), 7.73 (d, J = 8.5 Hz, 2H, H-2',6'), 10.12 (s,
1H, NH). 13
C NMR (DMSO-d6) δ 169.8, 155.3, 148.0, 141.2, 133.9, 133.7, 132.3, 129.6, 128.7,
127.5, 126.9, 122.1, 37.7, 33.2. EIMS m/z 348 [M+2] (34), 346 [M]+
(100), 345 (40), 305 (11), 303
(33), 140 (18), 137 (11), 102 (14), 96 (9), 75 (15), 69 (11). Anal. Calcd for C16H11ClN2OS2: C,
55.40; H, 3.20; N, 8.08. Found: C, 55.47; H, 3.23; N, 8.02.
4.1.3.36. 3-(3-Fluorophenyl)-6,7-dihydro-7-(3-thienyl)-isothiazolo[4,5-b]pyridin-5(4H)-one (9e).
Yellowish solid, mp 166–168 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.90 (dd, J = 6.4 Hz, J = 15.6
Hz, 1H, CH2), 2.94 (dd, J = 8.4 Hz, J = 15.6 Hz, 1H, CH2), 4.85 (dd, J = 6.4 Hz, J = 8.4 Hz, 1H,
CH), 7.16 (dd, J = 1.2 Hz, J = 5.0 Hz, 1H, H-4''), 7.31 (m, 1H, HAr), 7.36 (d, J = 2.8 Hz, 1H, H-2''),
7.49 (m, 1H, HAr), 7.54 (m, 2H, HAr), 7.59 (dd, J = 2.8 Hz, J = 5.0 Hz, 1H, H-5''), 10.18 (s, 1H, NH).
13
C NMR (DMSO-d6) δ 169.8, 163.1, 161.2, 155.0, 148.1, 141.2, 135.5, 135.4, 133.7, 130.8, 130.7,
127.5, 126.9, 124.0, 122.1, 116.1, 116.0, 114.7, 114.5, 37.7, 33.2. EIMS m/z 330 [M]+
(1), 121 (17),
109 (16), 96 (24), 95 (28), 94 (16), 75 (22), 71 (29), 70 (31), 69 (36), 45 (100). Anal. Calcd for
C16H11FN2OS2: C, 58.16; H, 3.36; N, 8.48. Found: C, 58.26; H, 3.39; N, 8.40.
4.1.3.37. 3-(4-Fluorophenyl)-6,7-dihydro-7-(3-thienyl)-isothiazolo[4,5-b]pyridin-5(4H)-one (9f).
Yellowish solid, mp 206–208 °C. 1
H NMR (DMSO-d6, 500 MHz): δ 2.89 (dd, J = 6.4 Hz, J = 15.6
Hz, 1H, CH2), 2.93 (dd, J = 8.4 Hz, J = 15.6 Hz, 1H, CH2), 4.83 (dd, J = 6.4 Hz, J = 8.4 Hz, 1H,
CH), 7.15 (d, J = 5.0 Hz, 1H, H-4''), 7.31 (t, J = 8.9 Hz, 2H, H-3',5'), 7.36 (d, J = 2.8 Hz, 1H, H-2''),
7.58 (dd, J = 2.8 Hz, J = 5.0 Hz, 1H, H-5''), 7.75 (dd, J = 5.6 Hz, J = 8.9 Hz, 2H, H-2',6'), 10.08 (s,
1H, NH). 13
C NMR (DMSO-d6) δ 169.8, 163.6, 161.7, 155.5, 147.8, 141.3, 133.5, 130.1, 130.0,
127.5, 126.9, 122.0, 115.7, 115.5, 37.7, 33.2. EIMS m/z 330 [M]+
(22), 287 (11), 140 (26), 122 (22),
121 (41), 109 (16), 96 (23), 95 (38), 94 (27), 75 (23), 71 (24), 70 (30), 69 (35), 45 (100). Anal.
Calcd for C16H11FN2OS2: C, 58.16; H, 3.36; N, 8.48. Found: C, 58.21; H, 3.38; N, 8.42.
4.2. Biology. Materials and methods
4.2.1. Phenotypic sea urchin embryo assay [11]
Adult sea urchins, Paracentrotus lividus L. (Echinidae), were collected from the
Mediterranean Sea on the Cyprus coast and kept in an aerated seawater tank. Gametes were obtained
by intracoelomic injection of 0.5 M KCl. Eggs were washed with filtered seawater and fertilized by
adding drops of diluted sperm. Embryos were cultured at room temperature under gentle agitation
with a motor-driven plastic paddle (60 rpm) in filtered seawater. The embryos were observed with a
Biolam light microscope (LOMO, St. Petersburg, Russia). For treatment with the test compounds, 5](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-27-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
26
mL aliquots of embryo suspension were transferred to six-well plates and incubated as a monolayer
at a concentration up to 2000 embryos/mL. Stock solutions of compounds were prepared in DMSO
at 10 mM concentration followed by a 10-fold dilution with 96% EtOH. This procedure enhanced
the solubility of the test compounds in the salt-containing medium (seawater), as evidenced by
microscopic examination of the samples. The maximal tolerated concentrations of DMSO and EtOH
in the in vivo assay were determined to be 0.05% and 1%, respectively. Higher concentrations of
either DMSO (≥0.1%) or EtOH (>1%) caused nonspecific alteration and retardation of the sea
urchin embryo development independent of the treatment stage. Combretastatin A-4 disodium
phosphate (CA4P, OXiGENE) served as a positive control. The antiproliferative activity was
assessed by exposing fertilized eggs (8–15 min after fertilization, 45–55 min before the first mitotic
cycle completion) to 2-fold decreasing concentrations of the compound. Cleavage alteration and
arrest were clearly detected at 2.5–5.5 h after fertilization, when control embryos reached 8-cell and
early blastula stages, respectively. The effects were estimated quantitatively as an effective threshold
concentration, resulting in cleavage alteration and embryo death before hatching or full mitotic
arrest. At these concentrations all tested microtubule destabilizers caused 100% cleavage alteration
and embryo death before hatching, whereas at 2-fold lower concentrations the compounds failed to
produce any effect. For microtubule-destabilizing activity, the compounds were tested on free-
swimming blastulae just after hatching (8–10 h after fertilization), which originated from the same
embryo culture. Embryo spinning was observed after 15 min to 20 h of treatment, depending on the
structure and concentration of the compound. Both spinning and lack of forward movement were
interpreted to be the result of the microtubule-destabilizing activity of a molecule. Video
illustrations are available at http://www.chemblock.com. Both sea urchin embryo assay and DTP
NCI60 cell line activity data are available free of charge via the Internet at http://www.zelinsky.ru.
Experiments with the sea urchin embryos fulfill the requirements of biological ethics. The artificial
spawning does not cause animal death, embryos develop outside the female organism, and both
postspawned adult sea urchins and the excess of intact embryos are returned to the sea, their natural
habitat.
4.2.2. Cell lines and reagents
Human cancer cell lines NCI-H1975, MDA-MB-468, MDA-MB-231, CAOV-3, TOV-112D
and MDA-MB-435 were purchased from the ATCC. MDA-361/DYT2, a tumorigenic subclone of
the human breast carcinoma MDA-MB-361 [18], A2780, A2780/Cis, A2780/ADR, MOR,
MOR/CPR, and MOR/ADR cell lines were purchased from Sigma-Aldrich. Cell lines were
authenticated annually by short-tandem repeat analysis (Promega STR profiling service) and
routinely tested for mycoplasma contamination (ATCC). Each cell line was cultured in its standard](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-28-320.jpg)

![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
28
Ltd. (http://www.chemblock.com/). The authors thank the National Cancer Institute (NCI)
(Bethesda, MD, USA) for screening compounds 8 and 9 by the Developmental Therapeutics
Program at NCI (Anti-cancer Screening Program; http://dtp.cancer.gov).
Appendix A. Supplementary data
Supplementary data associated with this article can be found in the online version, at
........................ These data include NMR-spectra of synthesized compounds.
Graphical abstract
References
[1] A.S. Kiselyov, M.M. Semenova, V.V. Semenov, Bioorg. Med. Chem. Lett. 19 (2009) 1195–
1198. DOI: 10.1016/j.bmcl.2008.12.078.
[2] V. Berdini, T.R. Early, M.A. O'Brien, A.J. Woodhead, P.G. Wyatt, PCT Int. Appl. (2006).
WO 2006008545.
[3] B.O. Buckman, J.B. Nicholas, K. Emayan, S.D. Seiwert, S. Yuan, PCT Int. Appl. (2014).
WO 2014113485.
[4] A.S. Kiselyov, M.N. Semenova, N.B. Chernyshova, A. Leitao, A.V. Samet, K.A. Kislyi,
M.M. Raihstat, T. Oprea, H. Lemcke, M. Lantow, D.G. Weiss, N.N. Ikizalp, S.A.
Kuznetsov, V.V. Semenov, Eur. J. Med. Chem. 45 (2010) 1683–1697.
DOI:10.1016/j.ejmech.2009.12.072.
[5] B.V. Lichitsky, A.N. Komogortsev, R.M. Belyi, A.A. Dudinov, M.M. Krayushkin, Russ.
Chem. Bull. 58 (2009) 1538–1541.
[6] J.R. Beck, R.P. Gajewski, R.E. Hackler, US Patent US 4346094, 1982.
[7] V.V. Semenov, V.A. Rusak, E.M. Chartov, M.I. Zaretsky, L.D. Konyushkin, S.I. Firgang,
A.O. Chizhov, V.V. Elkin, N.N. Latin, V.M. Bonashek, O.N. Stas'eva, Russ. Chem. Bull. 56
(2007) 2448–2455.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-30-320.jpg)
![M
ANUSCRIPT
ACCEPTED
ACCEPTED MANUSCRIPT
29
[8] V.V. Semenov, A.S. Kiselyov, I.Y. Titov, I.K. Sagamanova, N.N. Ikizalp, N.B.
Chernysheva, D.V. Tsyganov, L.D. Konyushkin, S.I. Firgang, R.V. Semenov, I.B.
Karmanova, M.M. Raihstat, M.N. Semenova, J. Nat. Prod. 73 (2010) 1796–1802.
DOI: 10.1021/np1004278.
[9] H.V. Frost, Justus Liebigs Ann. Chem. 250 (1889) 156–166.
[10] K. Gewald, P. Bellmann, Lieb. Ann. Chem. (1979) 1534–1546.
[11] M.N. Semenova, A.S. Kiselyov, V.V. Semenov, BioTechniques 40 (2006) 765–774.
DOI: 10.2144/000112193.
[12] M.N. Semenova, A.S. Kiselyov, I.Y. Titov, M. Molodtsov, E. Grishchuck, I. Spiridonov,
V.V. Semenov, Chem. Biol. Drug. Des. 70 (2007) 485–490.
DOI: 10.1111/j.1747-0285.2007.00591.x.
[13] M.N. Semenova, A.S. Kiselyov, D.V. Tsyganov, L.D. Konyushkin, S.I. Firgang, R.V.
Semenov, O.R. Malyshev, M.M. Raihstat, F. Fuchs, A. Stielow, M. Lantow, A.A.
Philchenkov, M.P. Zavelevich, N.S. Zefirov, S.A. Kuznetsov, V.V. Semenov, J. Med. Chem.
54 (2011) 7138–7149. dx.doi.org/10.1021/jm200737s.
[14] https://dtp.cancer.gov/discovery_development/nci-60/cell_list.
[15] C.V. Do, A. Faouzi, C. Barette, A. Farce, M.-O. Fauvarque, E. Colomb, L. Catry, O.
Berthier-Vergnes, M. Haftek, R. Barret, Th. Lomberget, Bioorg. Med. Chem. Lett. 26 (2016)
174–180. DOI: 10.1016/j.bmcl.2015.11.010.
[16] S.J. Lee, H.J. Lee, D.H. Moon, Anticancer Res. 31 (2011) 2135–2140.
[17] https://dtp.cancer.gov/discovery_development/nci-60/cell_list.htm.
[18] D. Yang, C.T. Kuan, J. Payne, A. Kihara, A. Murray, L.M. Wang, M. Alimandi, J.H. Pierce,
I. Pastan, M.E. Lippman, Clin. Cancer Res. 4 (1998) 993–1004.](https://image.slidesharecdn.com/65f72170-883f-49a2-95cd-3f87aa55d71c-160926194953/85/Isothiazoles-2016-31-320.jpg)
