- Clinical pharmacokinetics applies pharmacokinetic principles to optimize drug therapy for individual patients. It helps enhance efficacy and reduce toxicity.
- The 4 basic principles are absorption, distribution, metabolism, and elimination which determine how the body processes a drug over time. Factors like solubility, vascularity and metabolism impact these principles.
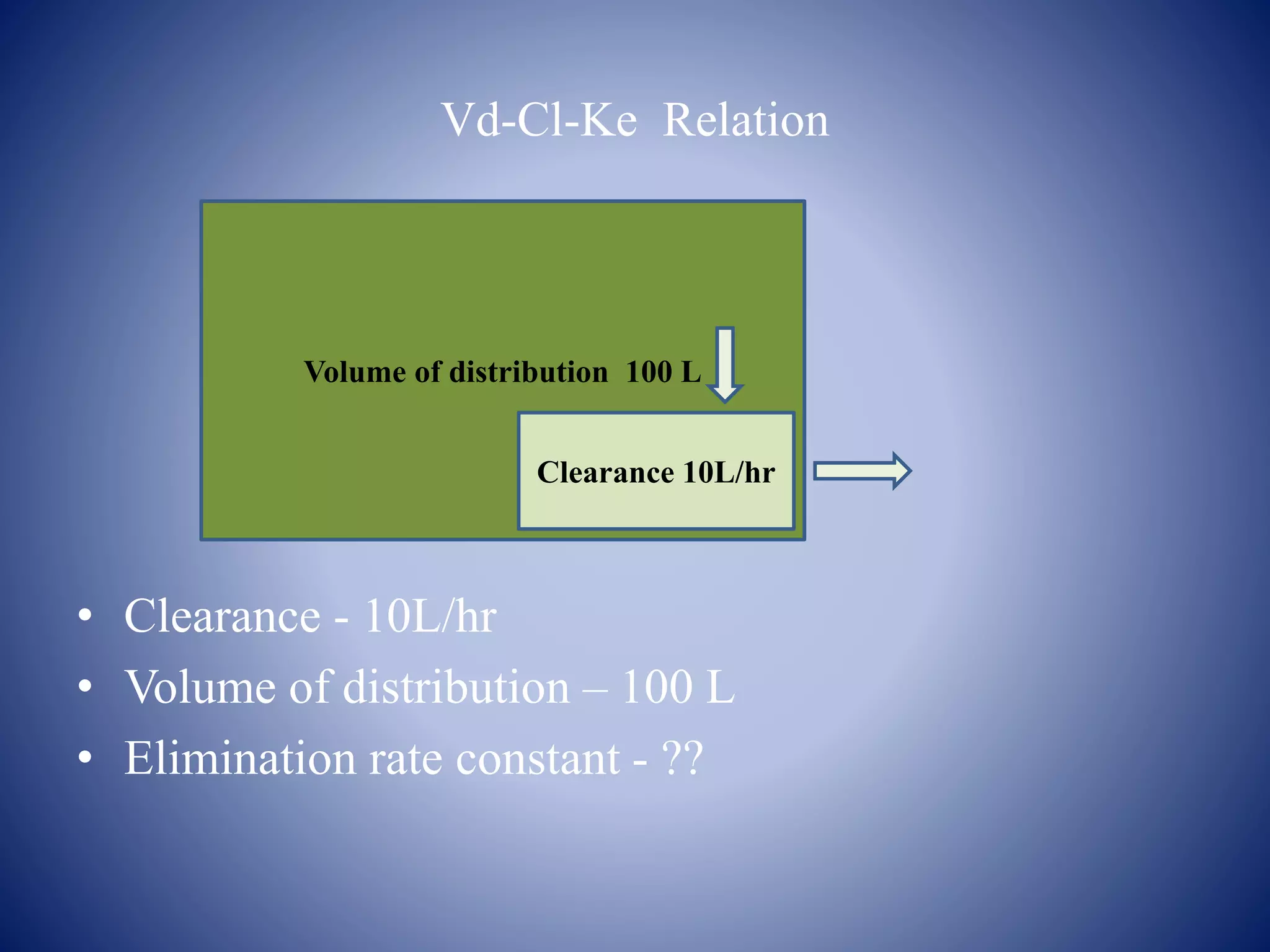
- Key pharmacokinetic parameters include volume of distribution, clearance, half-life, and bioavailability which describe the time course and extent of drug exposure in the body. Pharmacokinetic models can be used to analyze drug behavior.



































![• The child’s maintenance dose can be calculated from adult dose by
using the following equation :
Child’s Dose = SA of Child in m2 × Adult dose
1.73
Where 1.73 is surface area in m2 of an average 70 Kg adult.
• Since the surface area of a child is in proportion to the body weight
according to equation,
SA ( in m2 ) = Body weight (in Kg ) ^ 0.7
• The following relationship can also be written for child’s dose :
Child’s Dose = [ Weight of child in Kg ] ^ 0.7 × Adult dose
70](https://image.slidesharecdn.com/clinicalpharmackokinetics-200117100246/75/Clinical-pharmackokinetics-37-2048.jpg)









