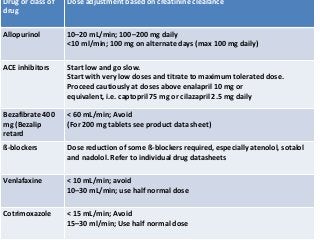
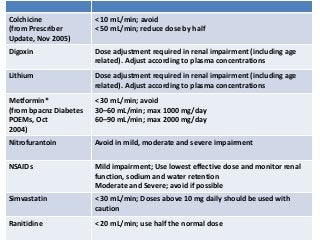
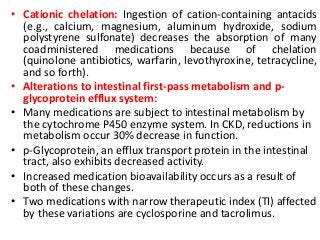
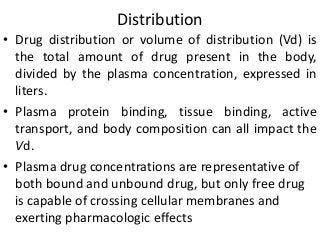
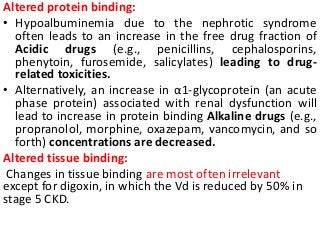
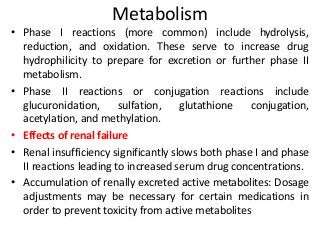
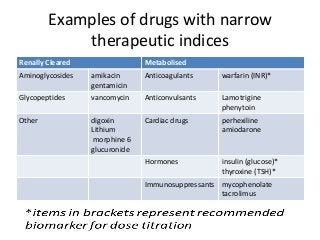
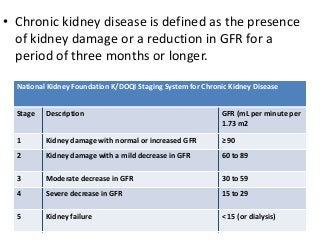
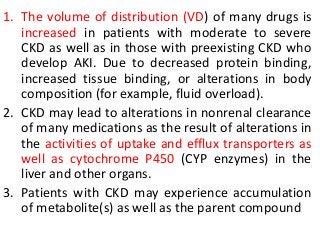
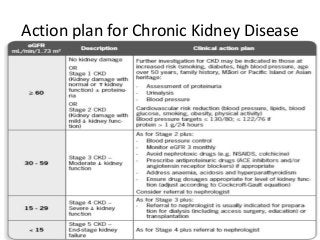
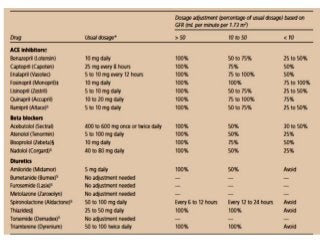
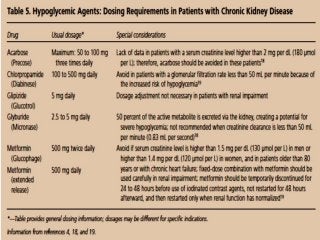
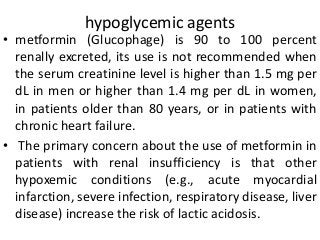
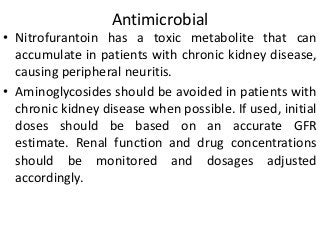
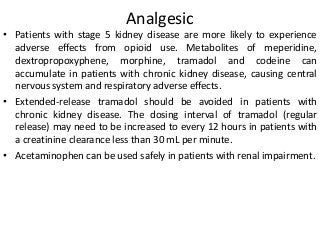
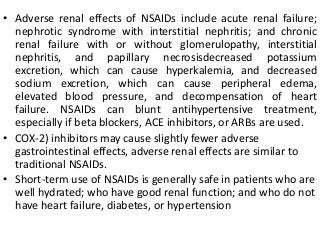
This document discusses drug dosing considerations in patients with chronic kidney disease. It provides guidelines for estimating glomerular filtration rate (GFR) using creatinine-based equations like CKD-EPI and Cockcroft-Gault to guide drug dosing. For drugs that are renally cleared, doses may need to be reduced as kidney function declines. Drugs are classified by their fraction excreted unchanged to determine dosing adjustments needed. Examples of common drug classes like antihypertensives, hypoglycemics, and antimicrobials are outlined with dosing recommendations based on a patient's GFR and kidney function stage. Measuring drug levels can help optimize therapeutic regimens in patients with chronic kidney






![CKD-EPI
• This CKD-EPI equation calculator should be used when Scr reported in
mg/dL. This equation is recommended when eGFR values above 60
mL/min/1.73 m2 are desired.
• GFR = 141 × min (Scr /κ, 1)α × max(Scr /κ, 1)-1.209 × 0.993Age × 1.018 [if
female] × 1.159 [if black]
• where:
• Scr is serum creatinine in mg/dL,
• κ is 0.7 for females and 0.9 for males,
• α is -0.329 for females and -0.411 for males,
• min indicates the minimum of Scr /κ or 1, and
• max indicates the maximum of Scr /κ or 1.
Calculator at:
https://www.niddk.nih.gov/health-information/health-communication-
programs/nkdep/lab-evaluation/gfr-calculators/adults-conventional-unit-ckd-
epi/Pages/default.aspx](https://image.slidesharecdn.com/drugdosing-161122000821/85/Drug-dosing-7-320.jpg?cb=1676626372)











![Fraction Excreted Unchanged
• The fraction excreted unchanged (fe) is the
proportion of the active drug cleared renally
in an average healthy person.
• The doses of drugs with fe ≥0.5 (50% or more
renally cleared) should usually be reduced in
patients with renal disease.
• patient dose =usual dose X
[(1-fe)+ fe X
𝑒𝑠𝑡𝑖𝑚𝑎𝑡𝑒𝑑 𝑝𝑎𝑡𝑖𝑒𝑛𝑡 𝑟𝑒𝑛𝑎𝑙 𝑓𝑢𝑛𝑐𝑡𝑖𝑜𝑛
𝑛𝑜𝑟𝑚𝑎𝑙 𝑟𝑒𝑛𝑎𝑙 𝑓𝑢𝑛𝑐𝑡𝑖𝑜𝑛)
]](https://image.slidesharecdn.com/drugdosing-161122000821/85/Drug-dosing-20-320.jpg?cb=1676626372)






![Loading dose:
Patient’s loading dose = Usual loading dose X (Patient’s VDÞ/Normal
VDÞ).
• Most published guidelines do not recommend a loading dose,
despite the well-documented evidence of altered VD of several
drugs in CKD patients.
• Loading doses may be required if a drug has a long half-life and
there is a need to rapidly achieve the desired steady-state
concentrations.
• Furthermore, if the VD of a drug is significantly increased in CKD
patients, a loading dose will likely be needed even if one was not
routinely recommended for those with normal renal function.
Usual Loading dose = Vd × IBW × Cp
• (Vd [L/kg]; IBW [ideal body weight; kg]; Cp [desired plasma
concentration; mg/L])](https://image.slidesharecdn.com/drugdosing-161122000821/85/Drug-dosing-29-320.jpg?cb=1676626372)




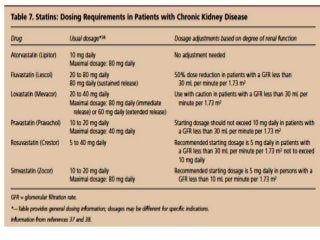
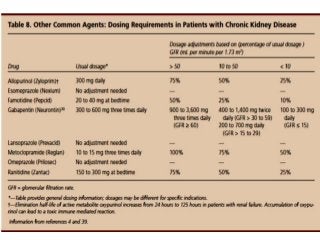
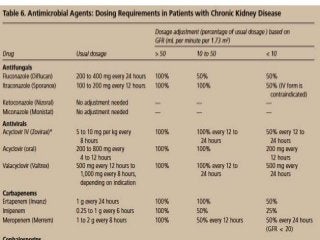
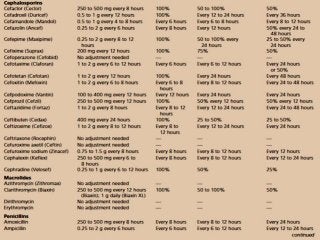











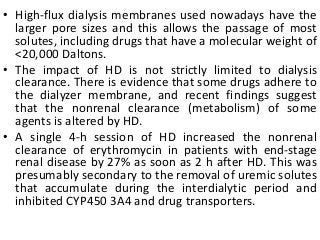
![Assessment of the impact of HD
• The most common method for assessing the
effect of HD is to calculate the dialyzer
clearance
• CLb
D= Qb[(Ab-Vb)/Ab],
• where Qb is blood flow through the dialyzer,
Ab is the concentration of drug in blood going
into the dialyzer, and Vb is the blood
concentration of drug leaving the dialyzer.](https://image.slidesharecdn.com/drugdosing-161122000821/85/Drug-dosing-61-320.jpg?cb=1676626372)



![5. Another alternative is to calculate the ‘total creatinine clearance’
(CLcr) based on the addition of the patient’s residual renal clearance
and expected extracorporeal clearance. This value can then be used
to estimate a maintenance dosing regimen based on medication
dosing guidelines specified for that resultant total CLcr range. Using
this method, most drugs will fall in the CLcr 25–50 ml/min range
6. A fourth method starts with the dose and dosing interval for a
patient with a GFR<10 ml/min (anuric dose), and makes dosage
adaptations based on the drug fraction expected to be removed by
extracorporeal therapy (FrEC)
i. Maintenance dose=anuric dose/[1- FrEC]
ii. Dosing interval=anuric dosing interval x [1-FrEC]
7. A fifth method starts with a normal dose (Dn) and reduces dose
based on normal clearance (Clnorm), non-renal clearance (Clnonrenal),
effluent rate (Qeff), and sieving coefficient (SC)
b. Dose =Dosenx[Clnonrenal+(QeffxSC)]/Clnorm
8. CRRT and EDD education should be an integral part of critical care
and nephrology fellowship training programs](https://image.slidesharecdn.com/drugdosing-161122000821/85/Drug-dosing-65-320.jpg?cb=1676626372)