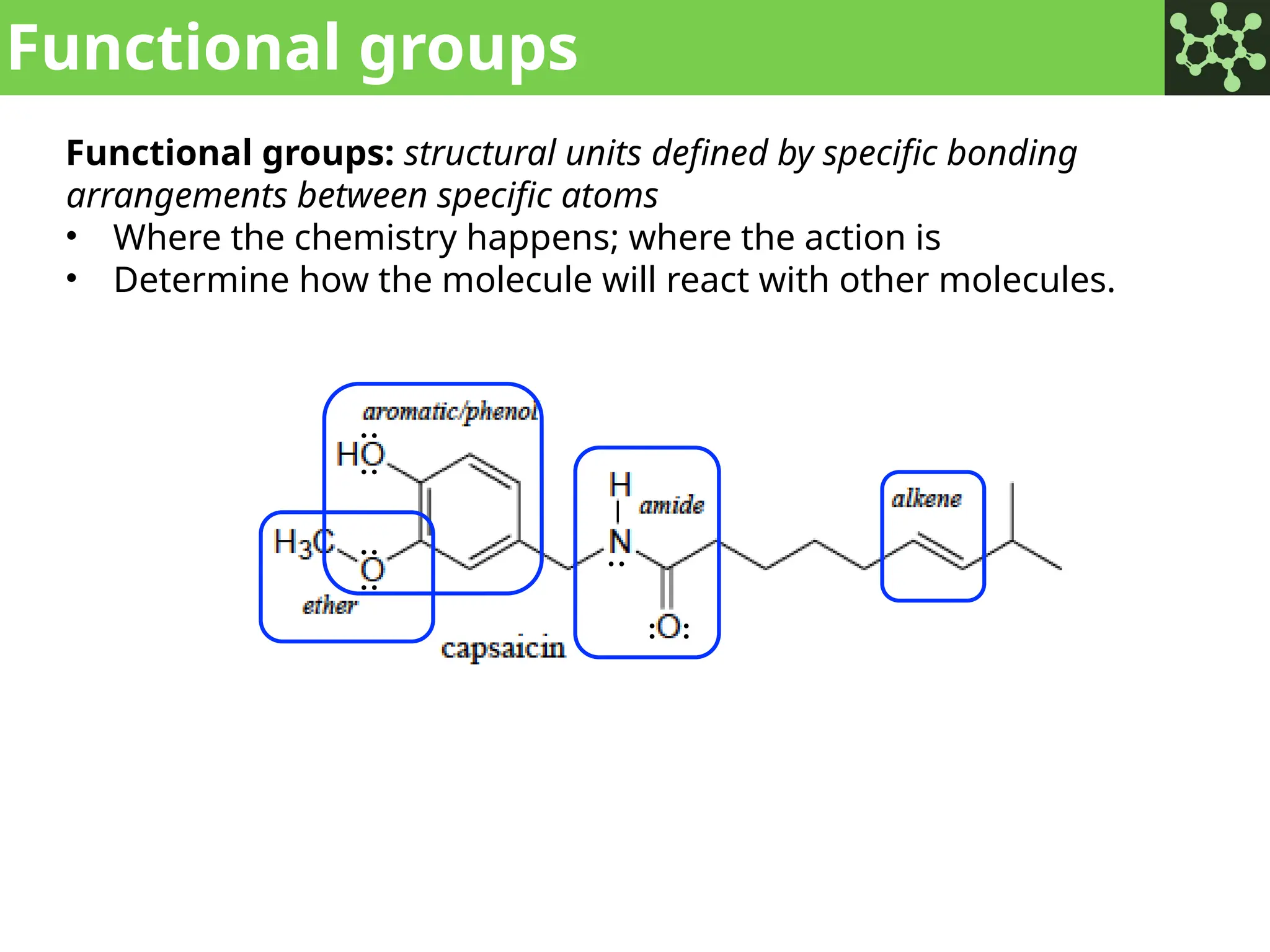
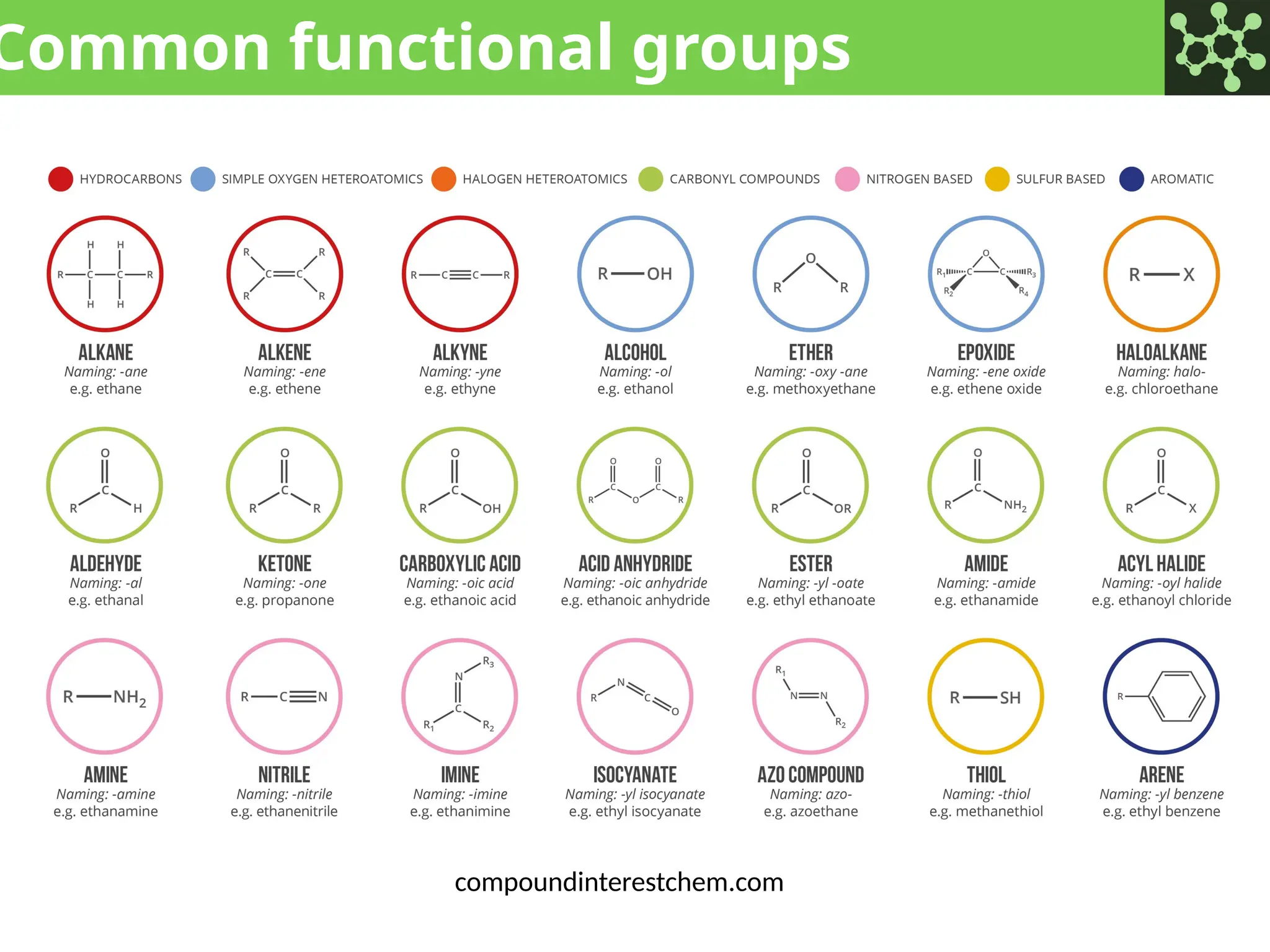
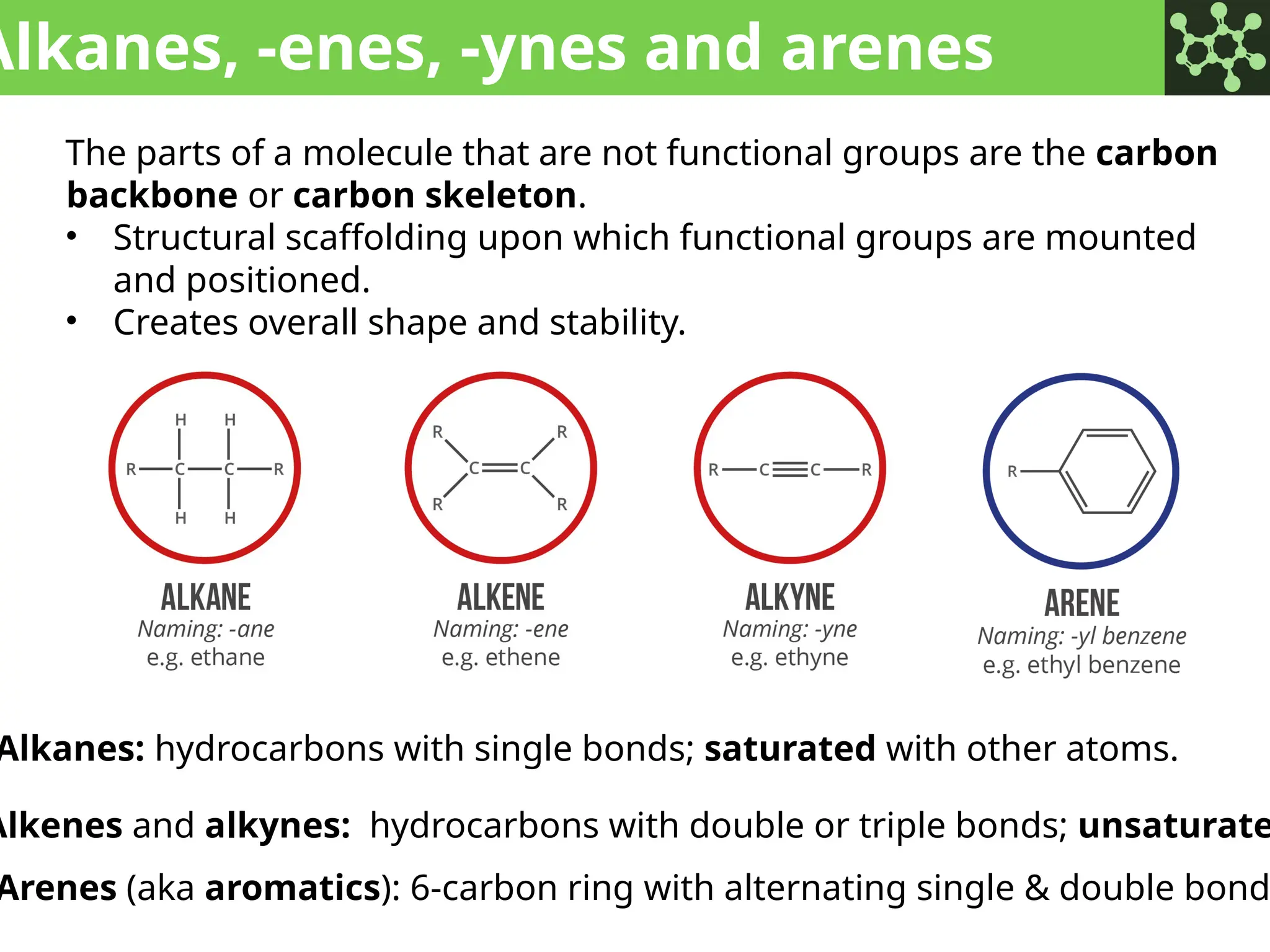
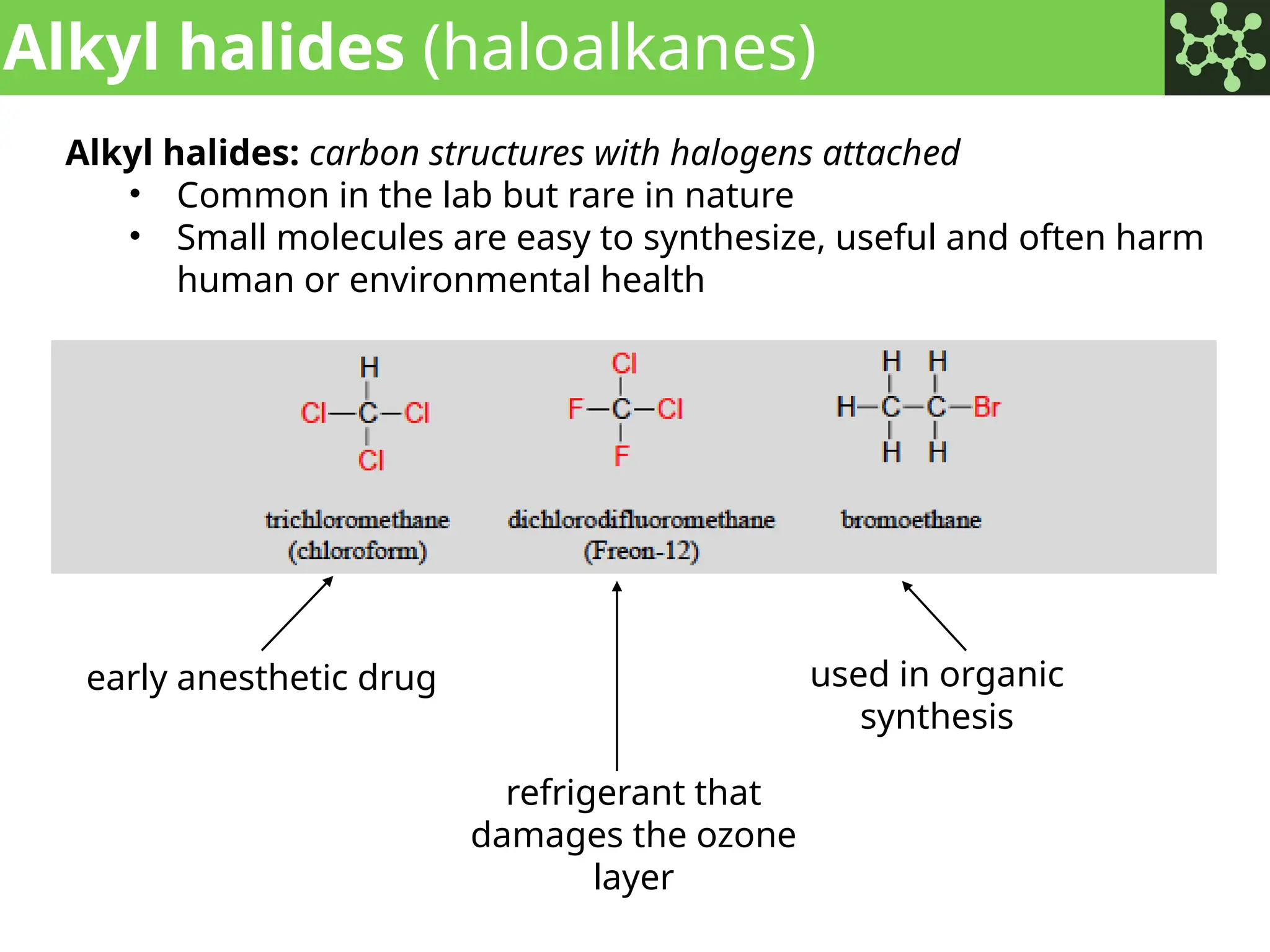
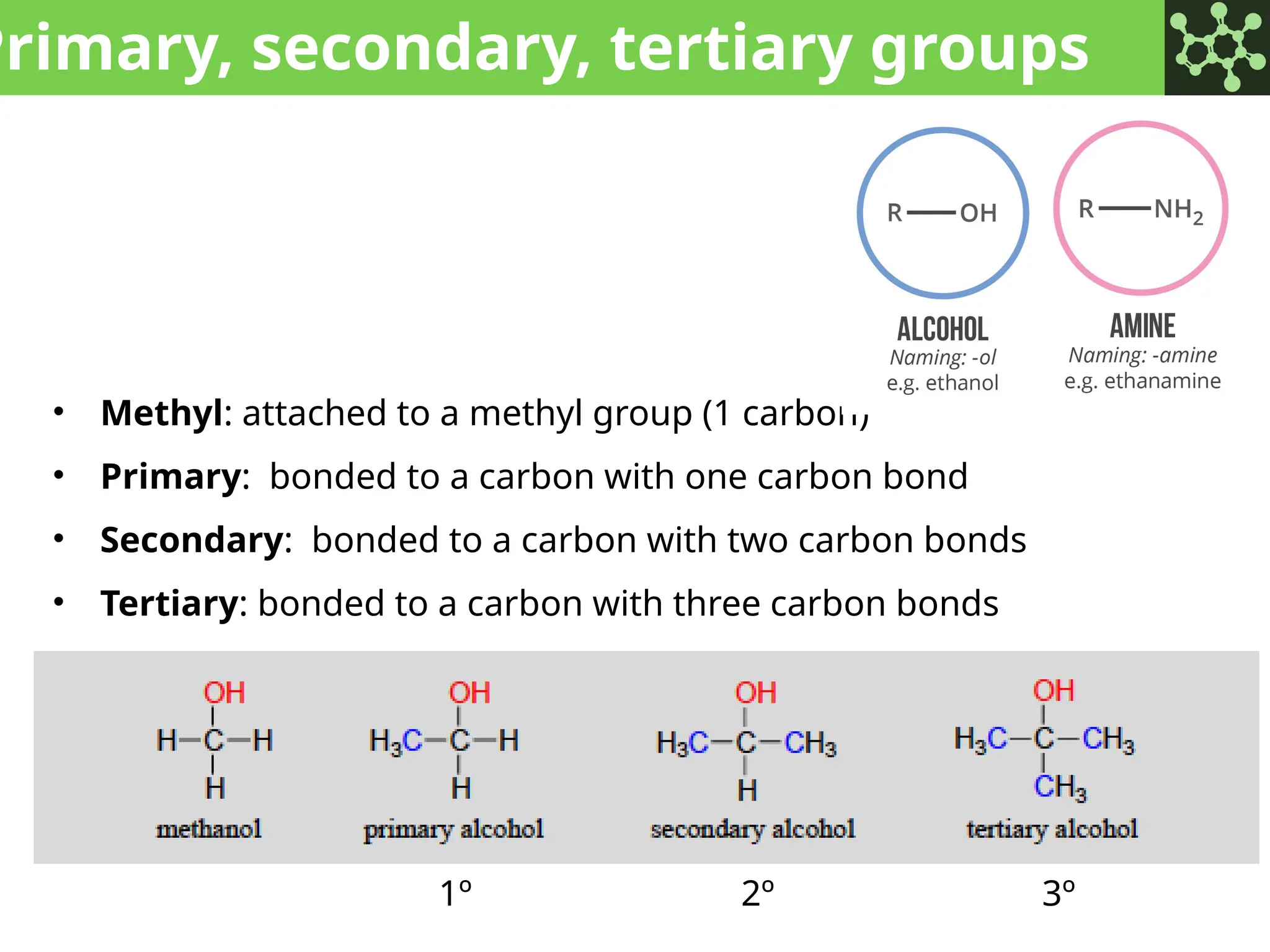
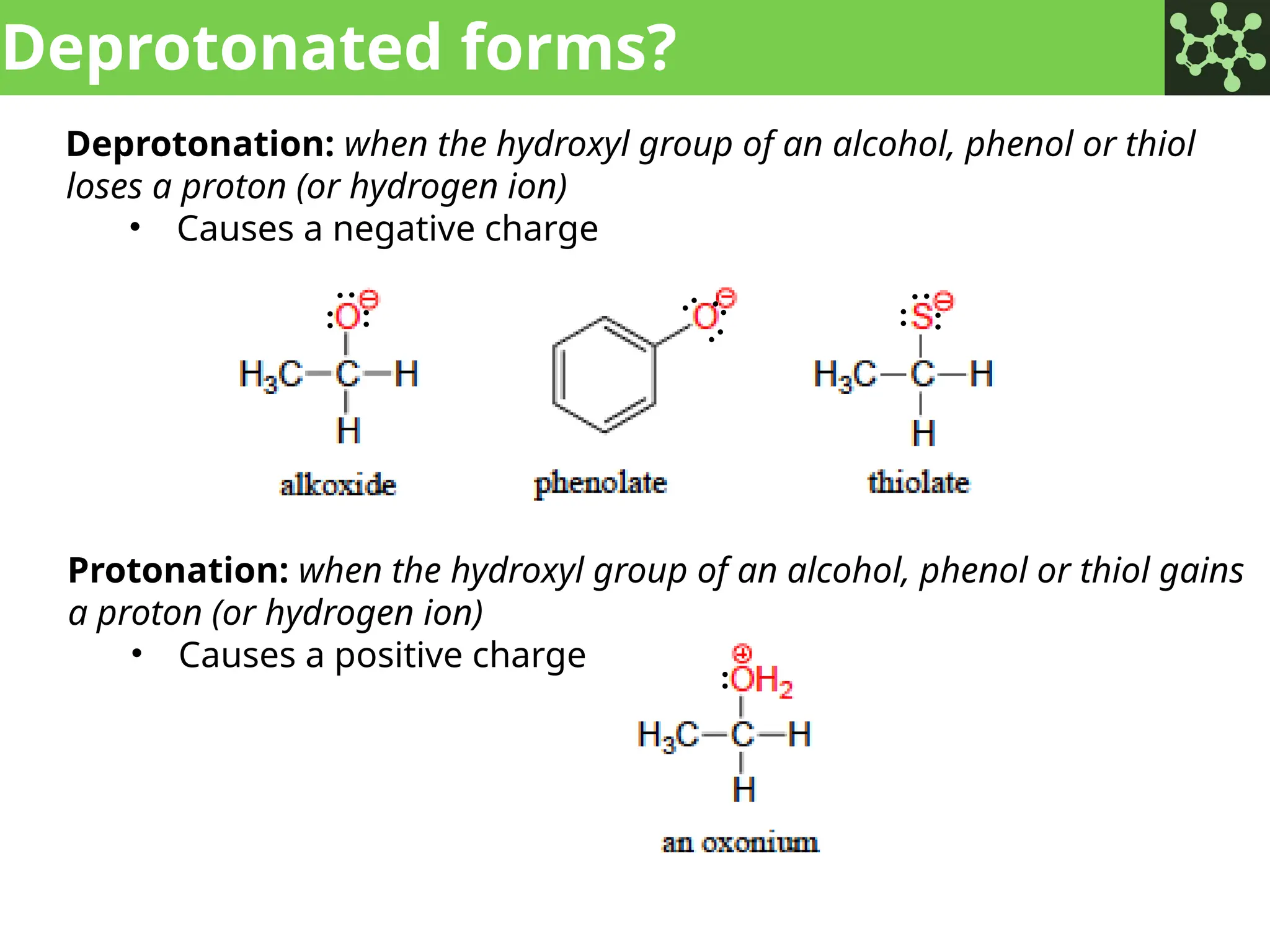
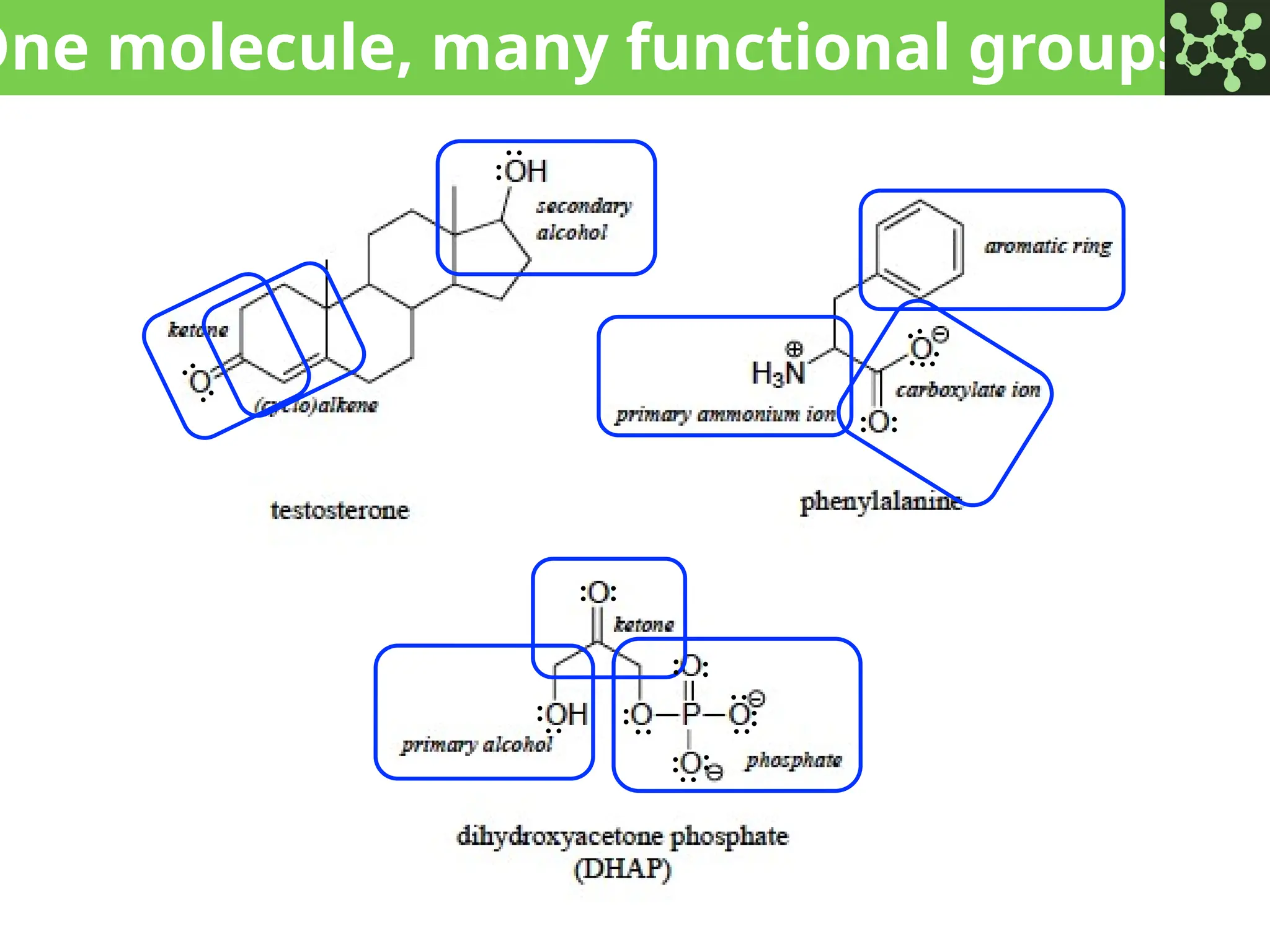
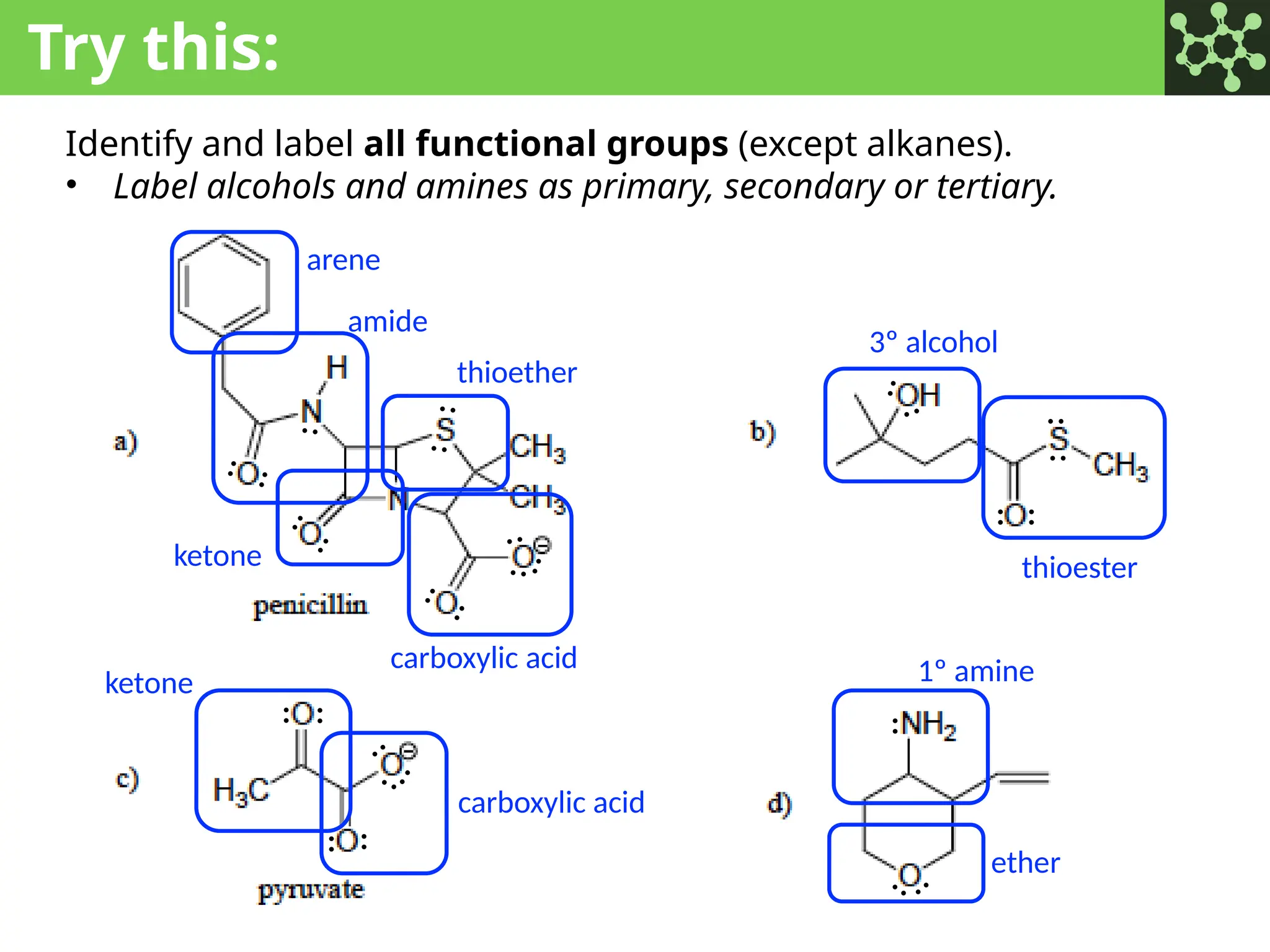
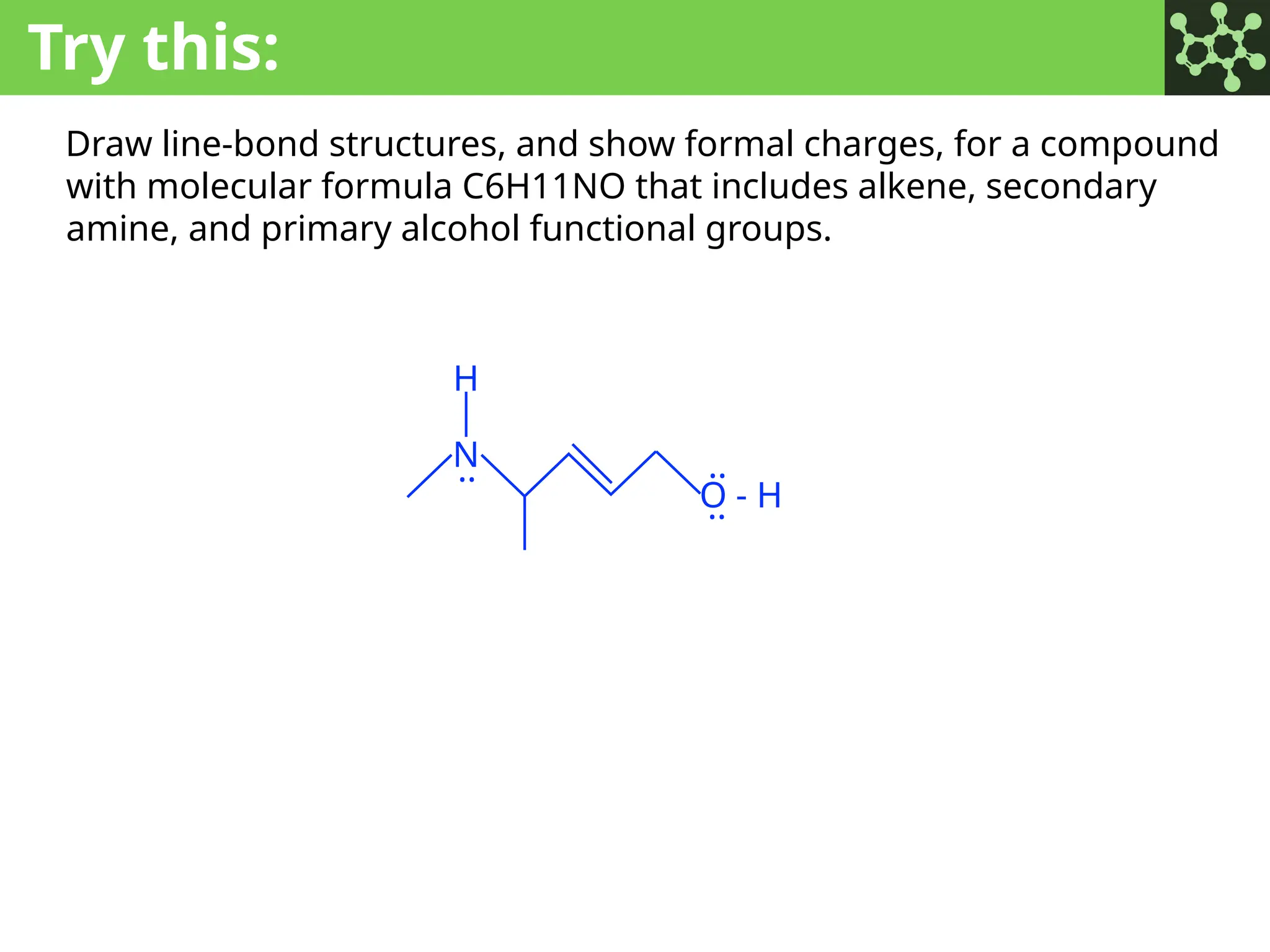
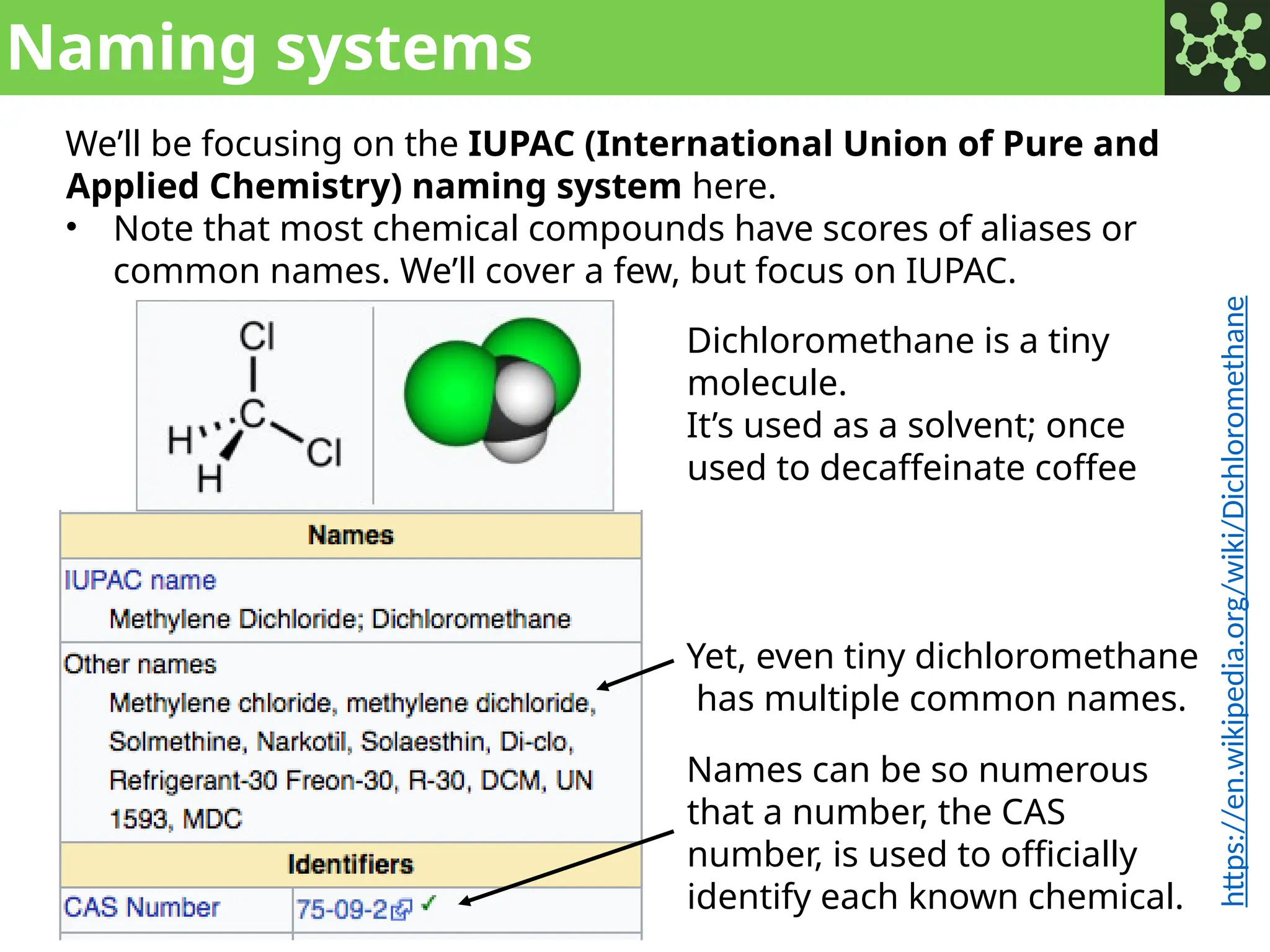
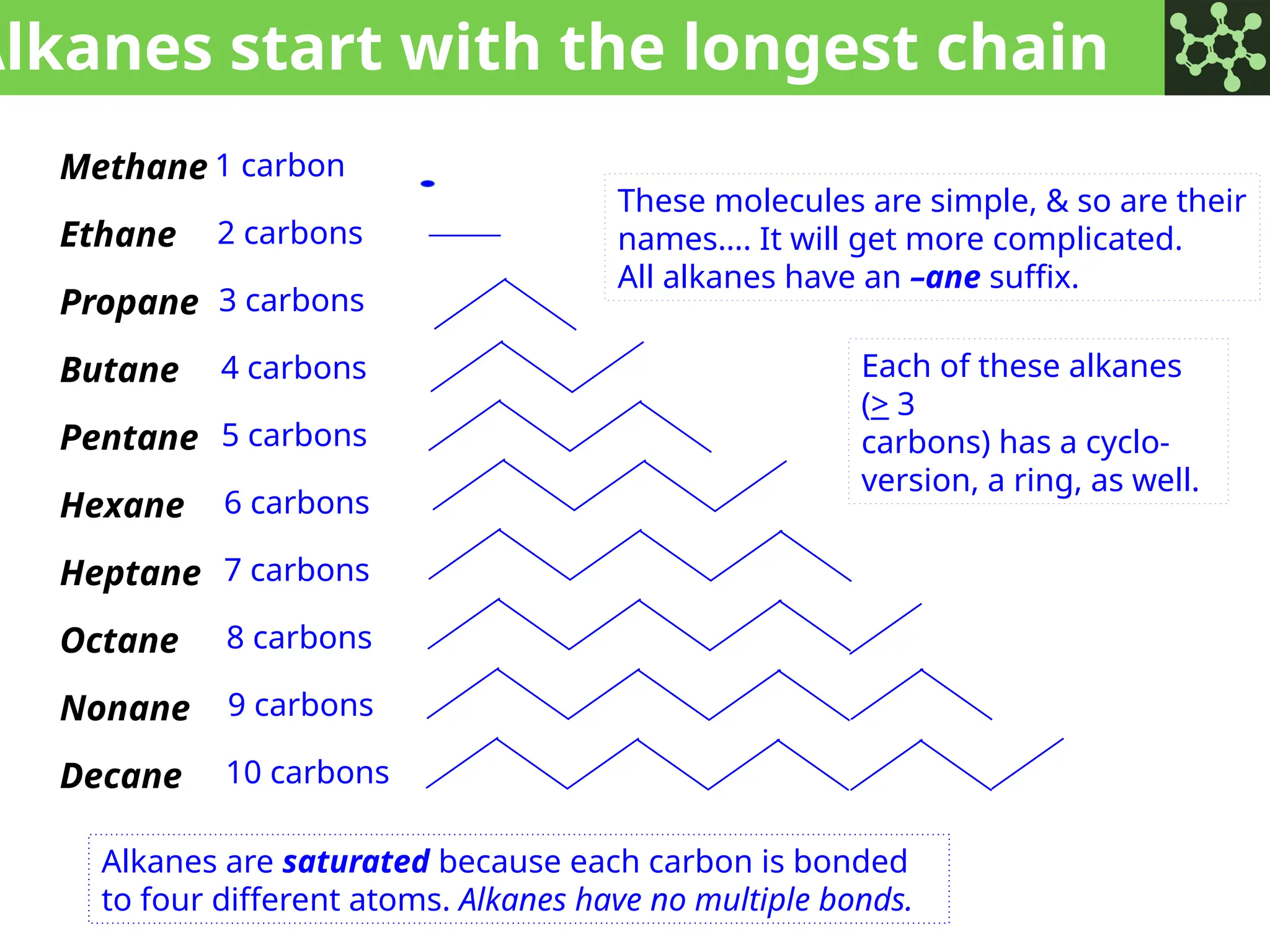
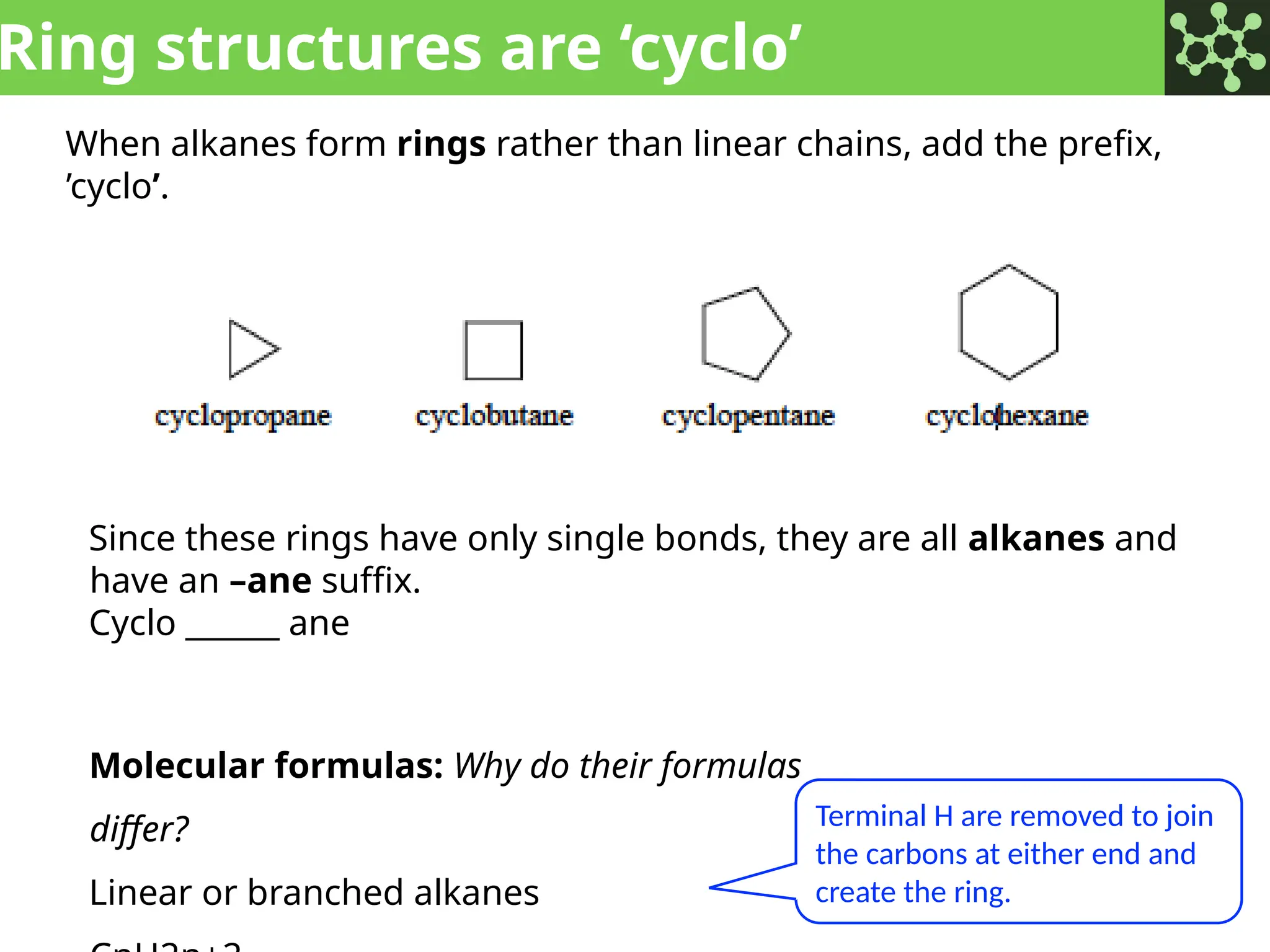
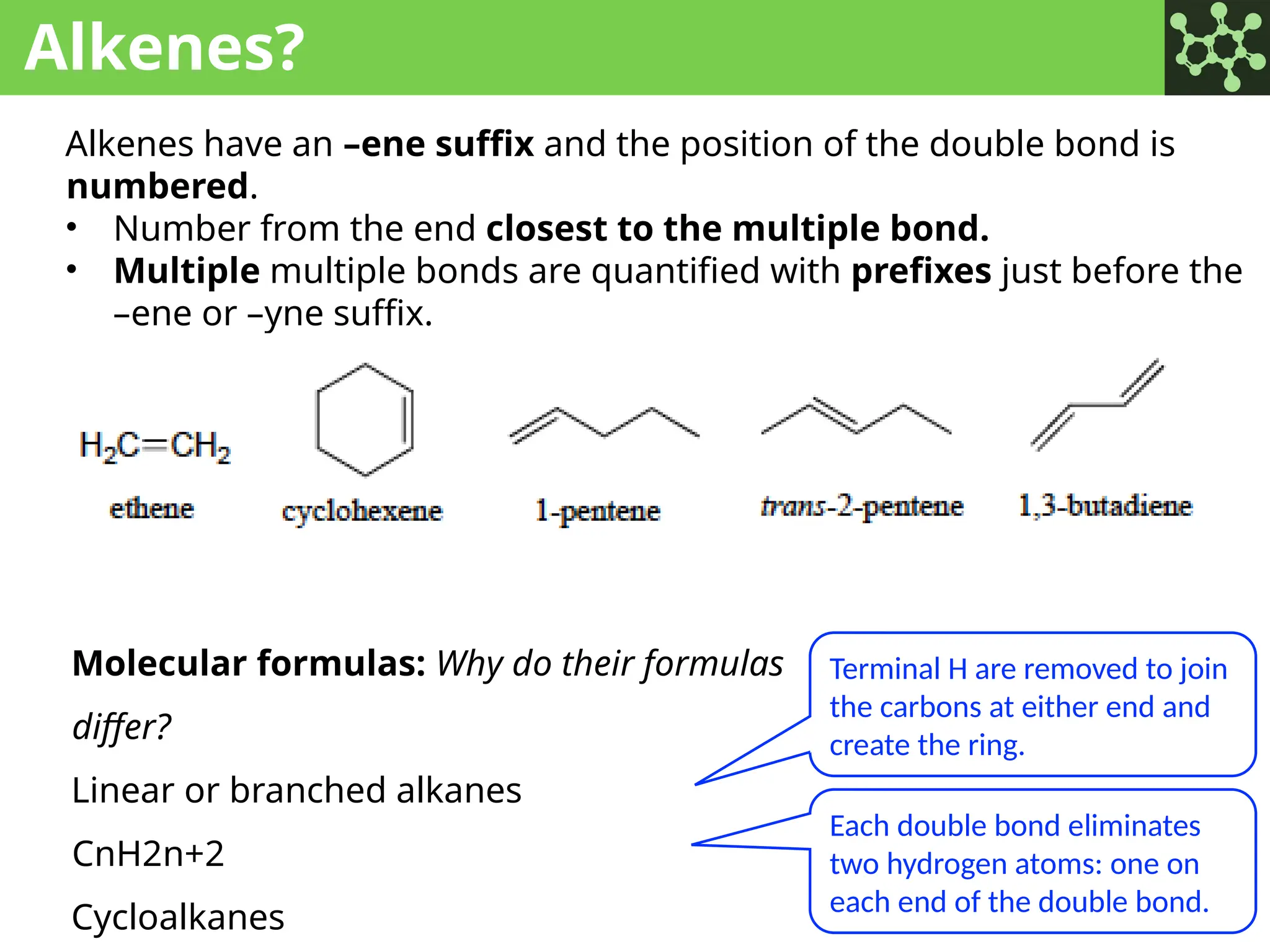
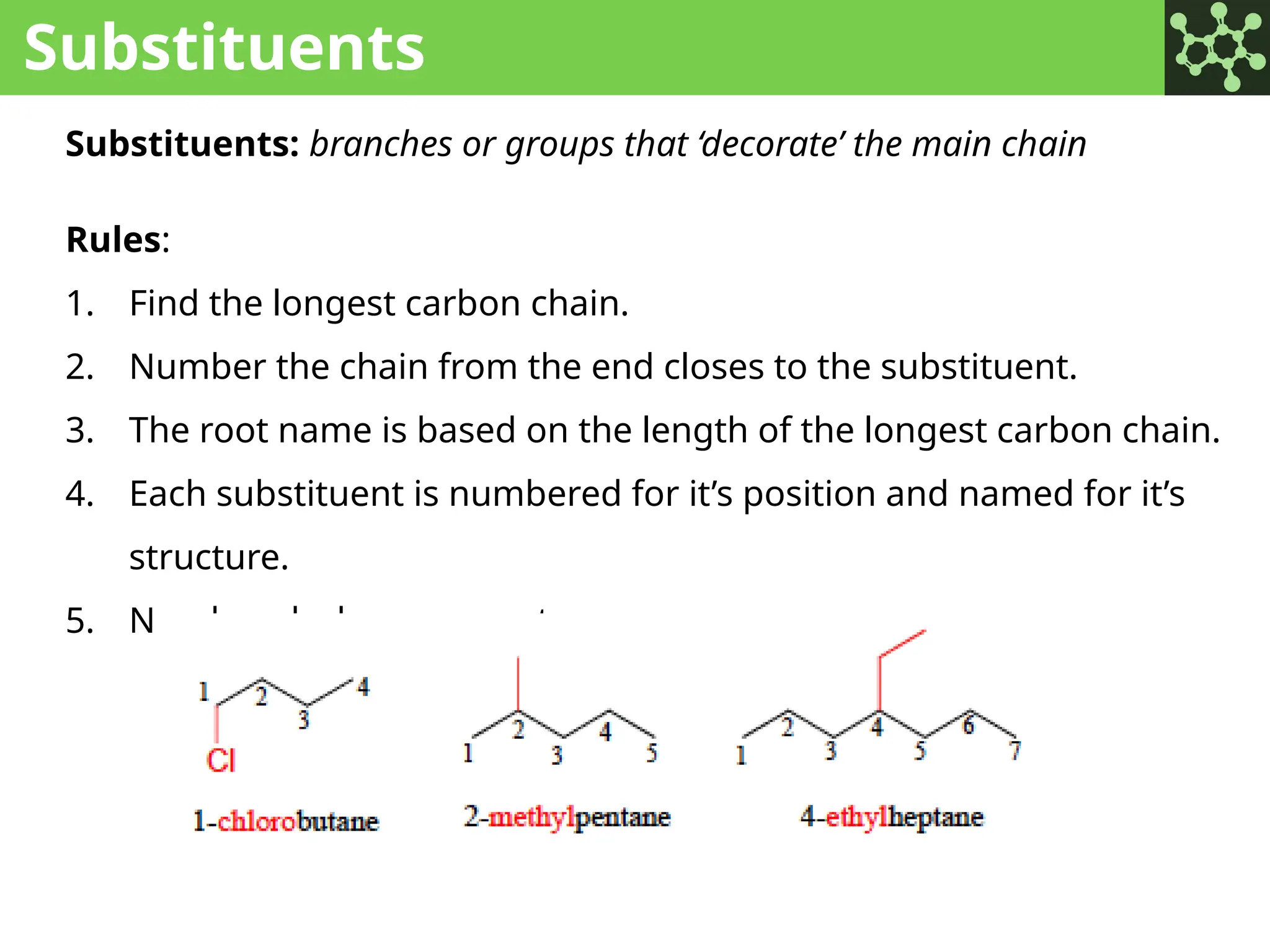
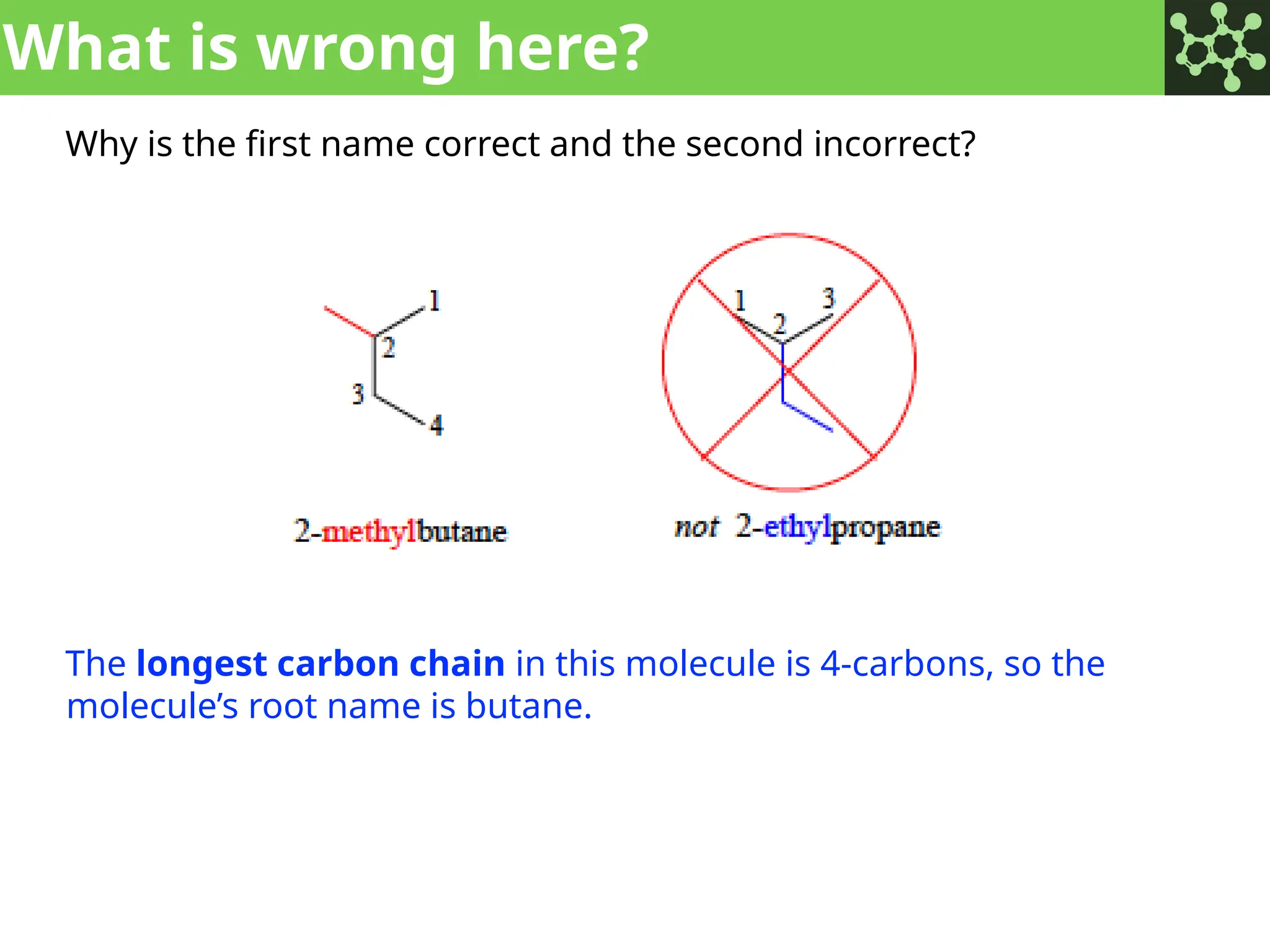
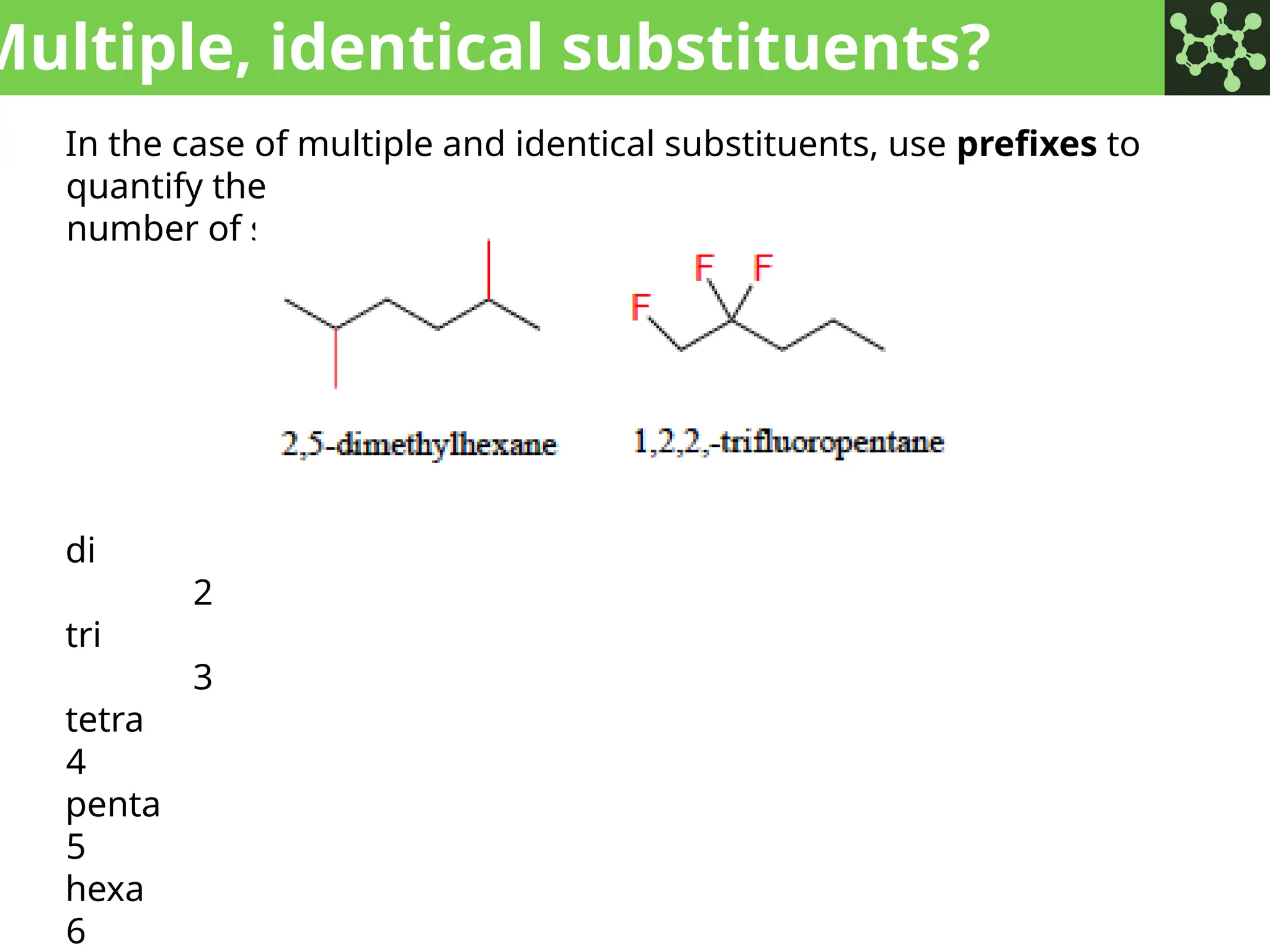
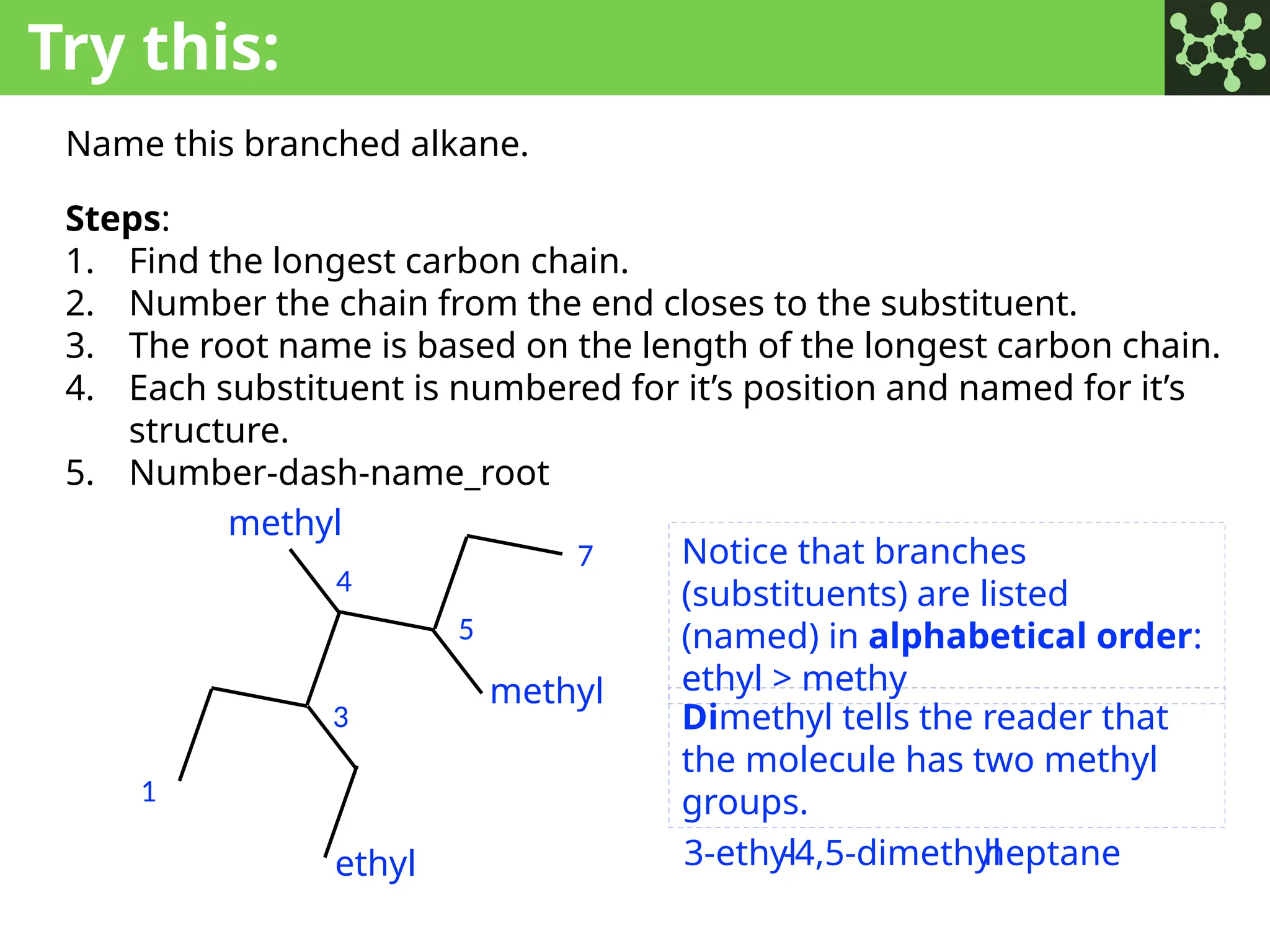
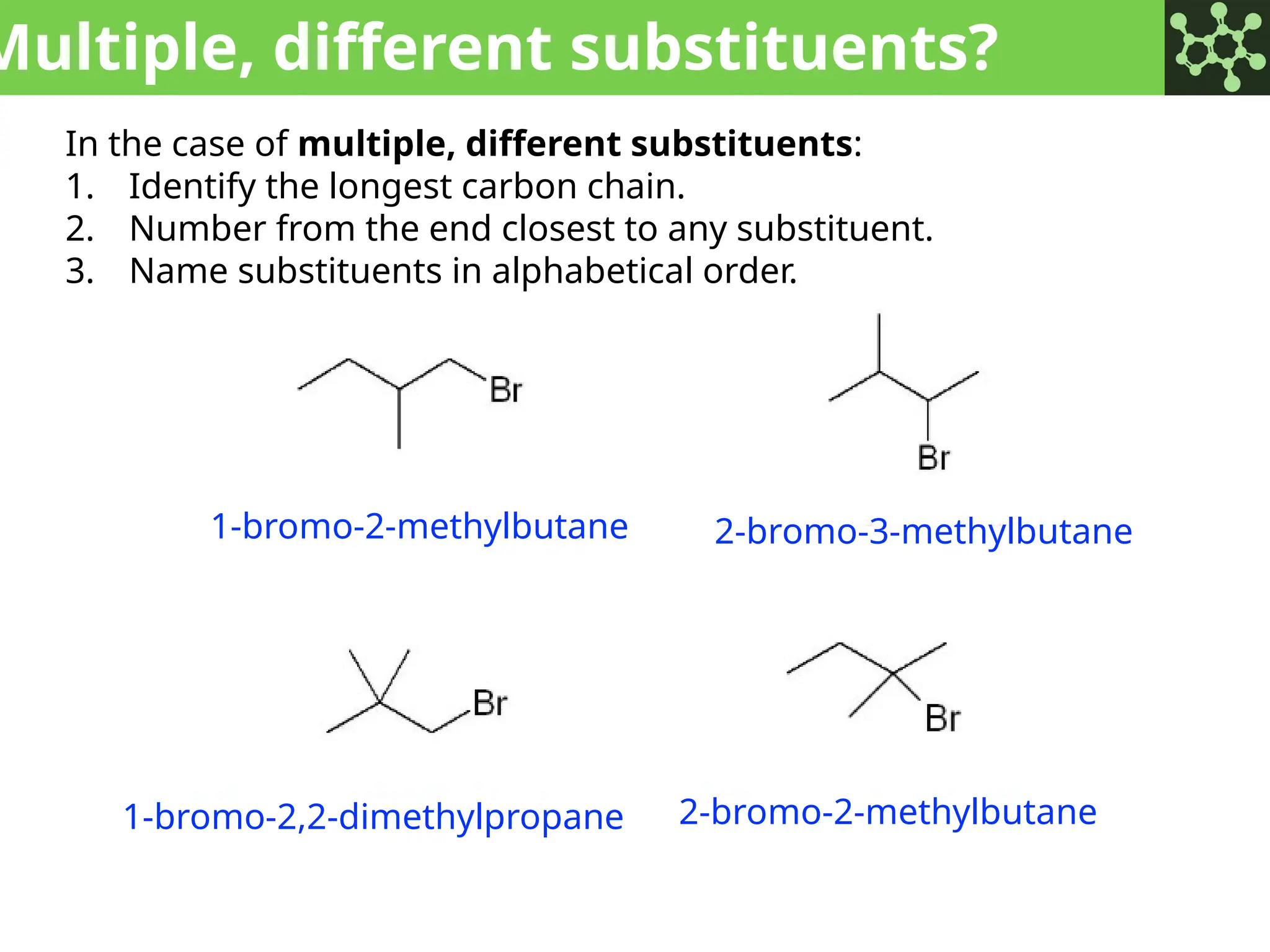
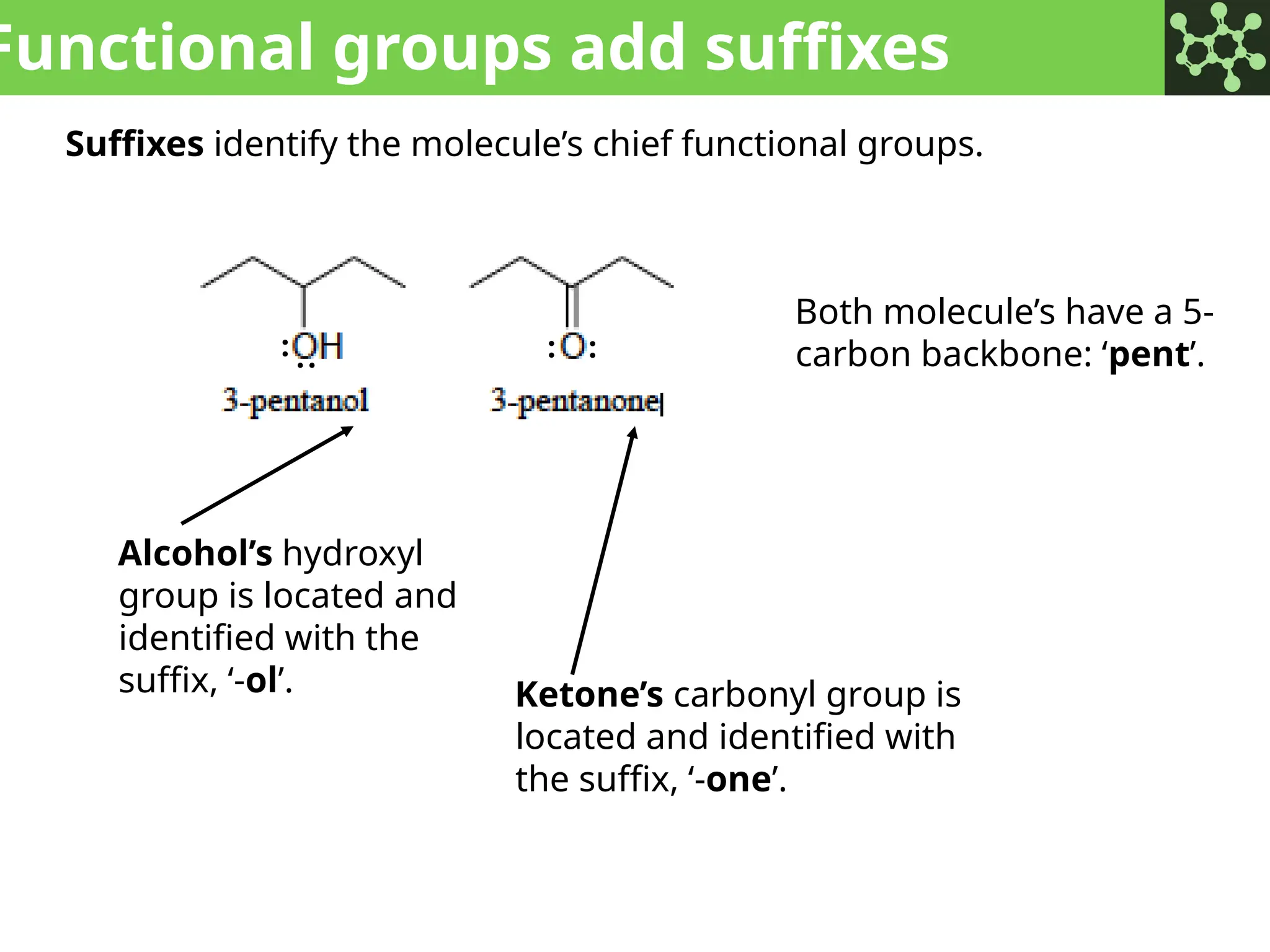
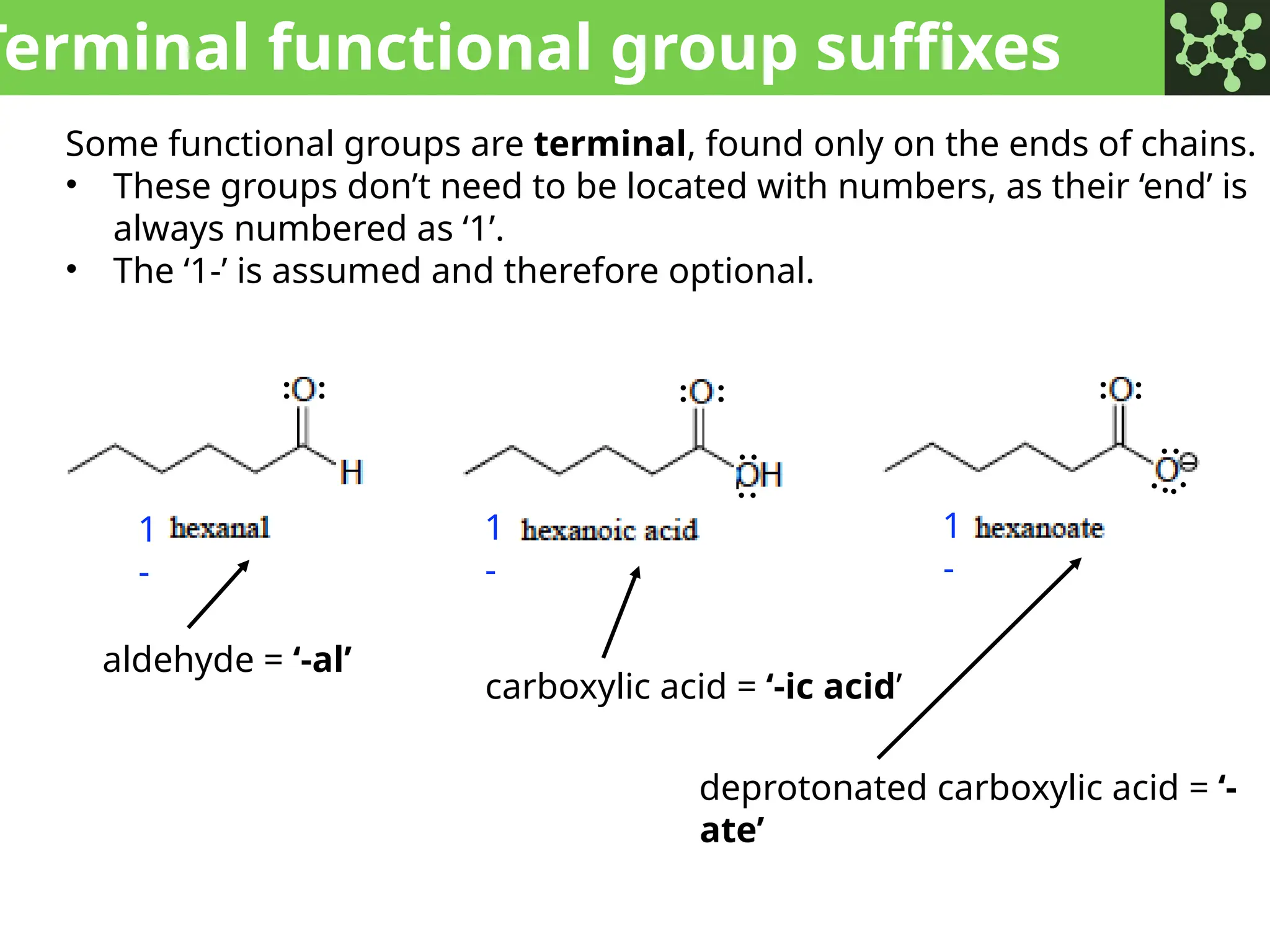
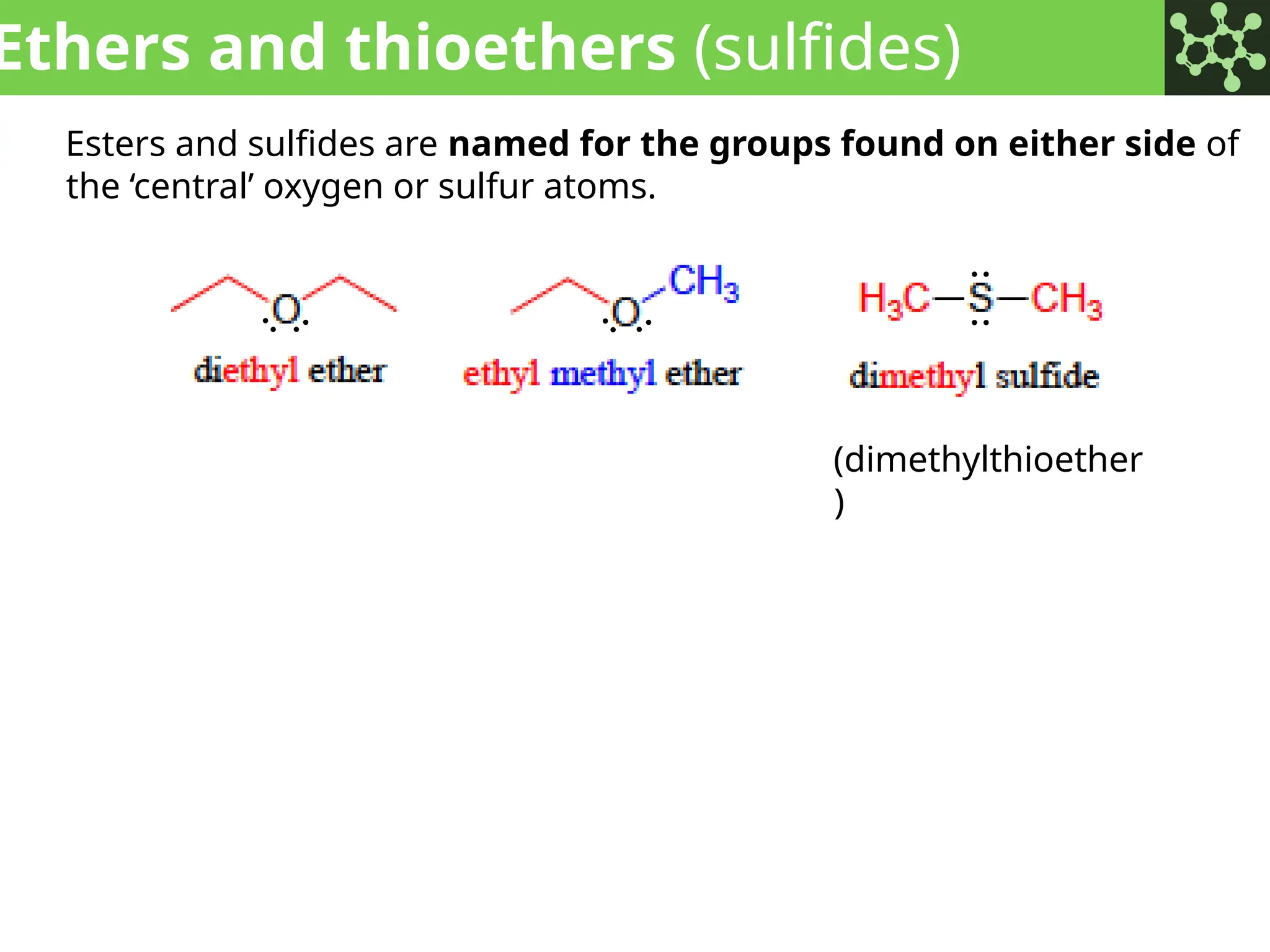
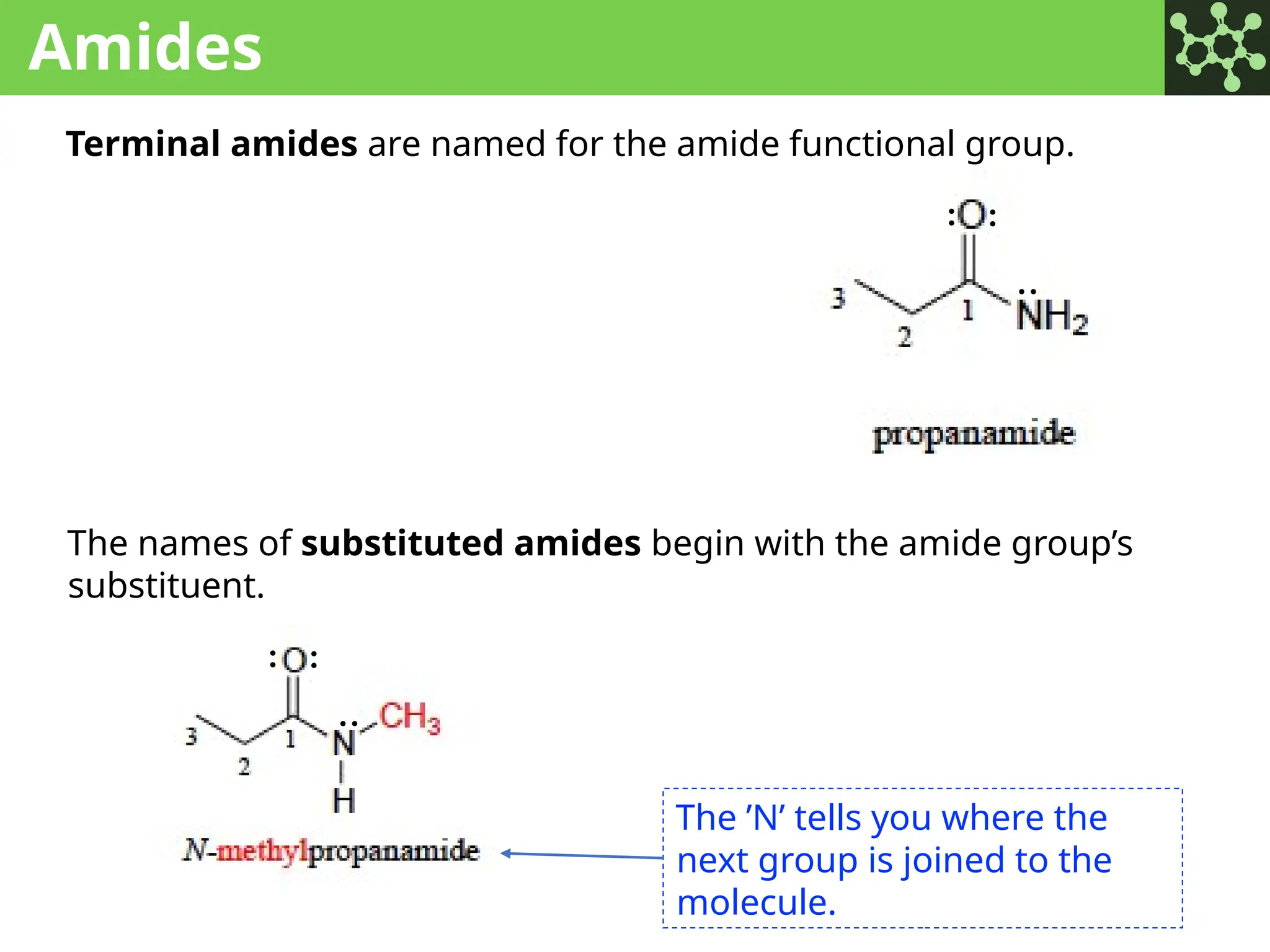
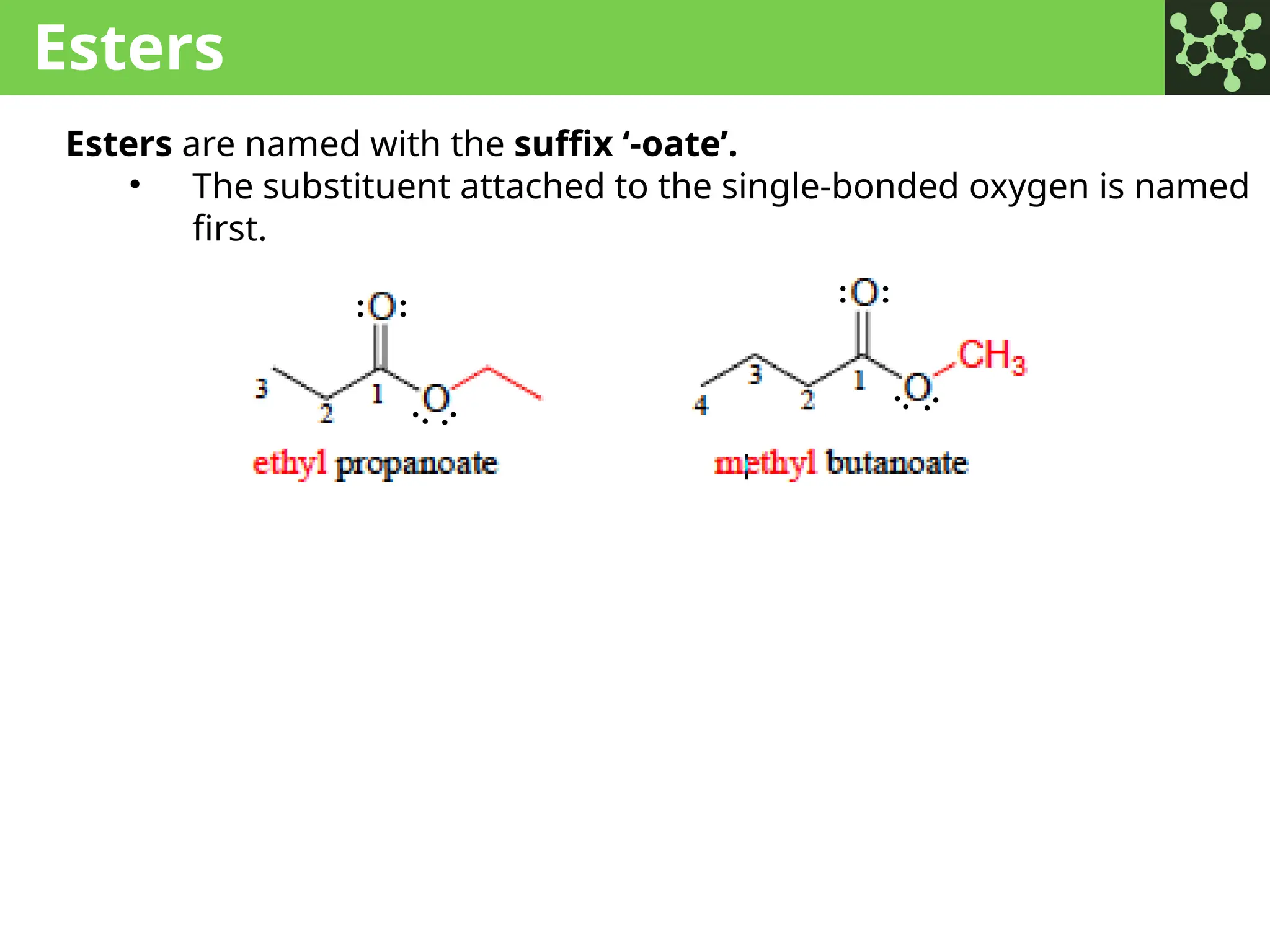
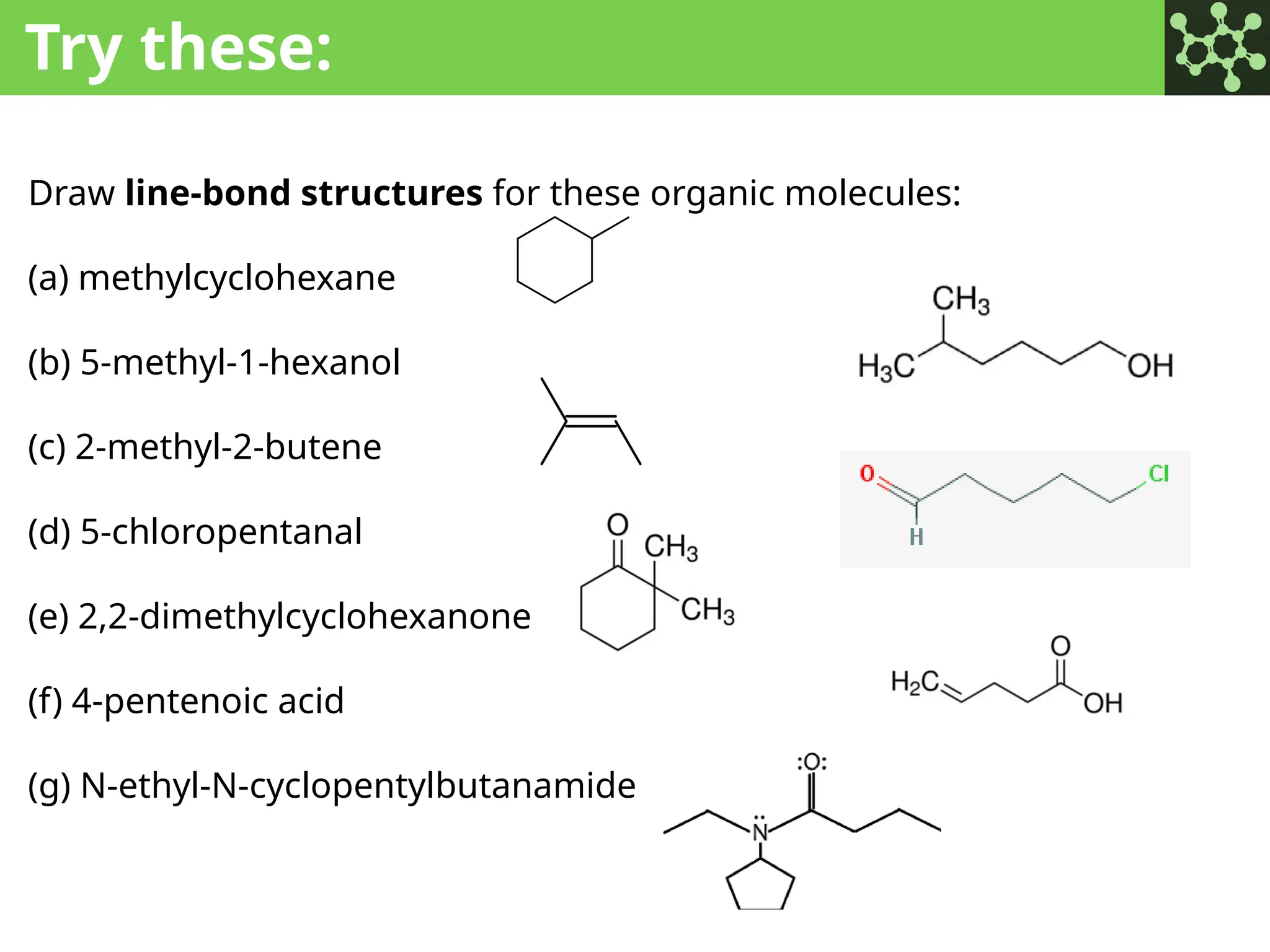
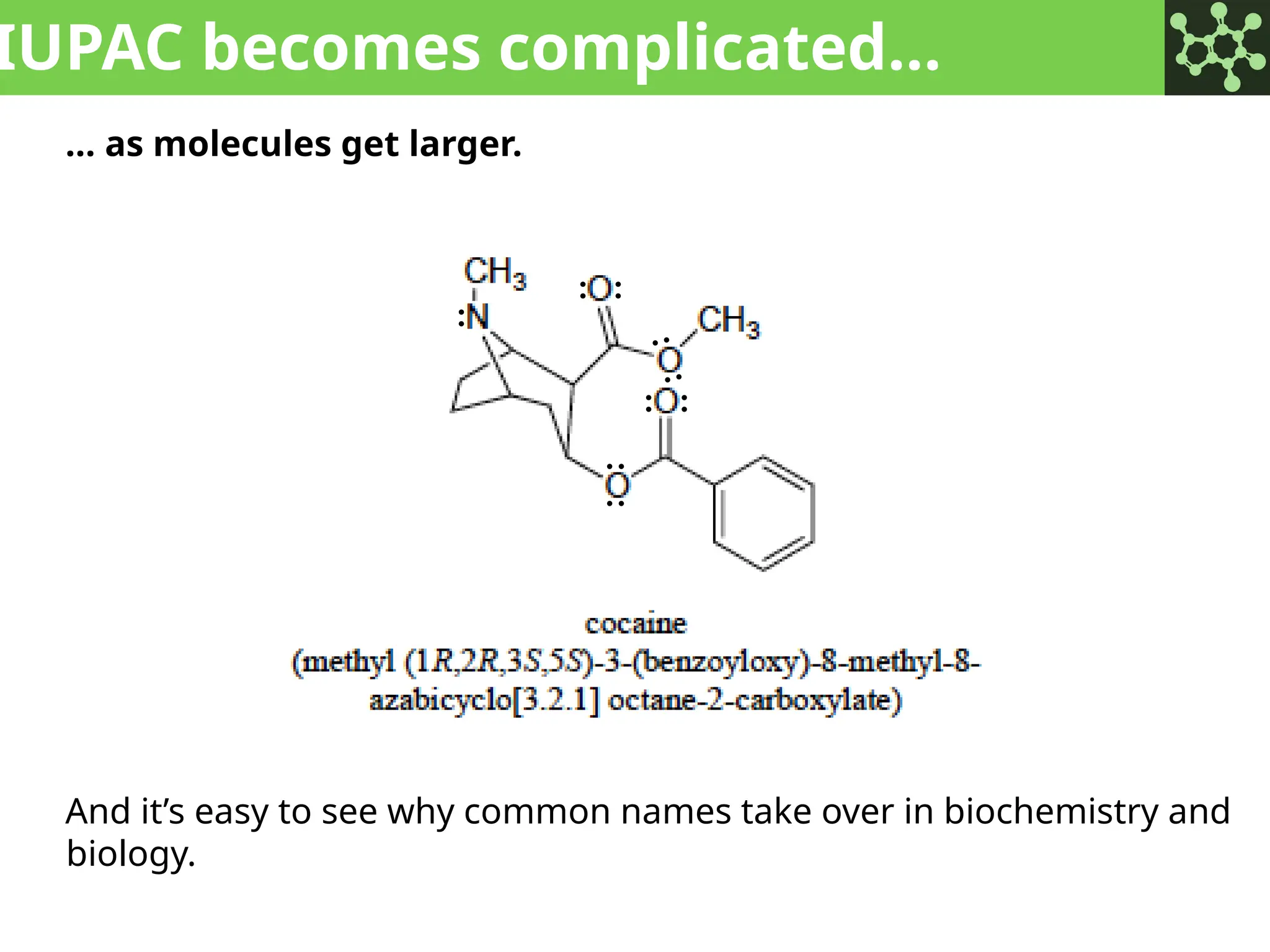
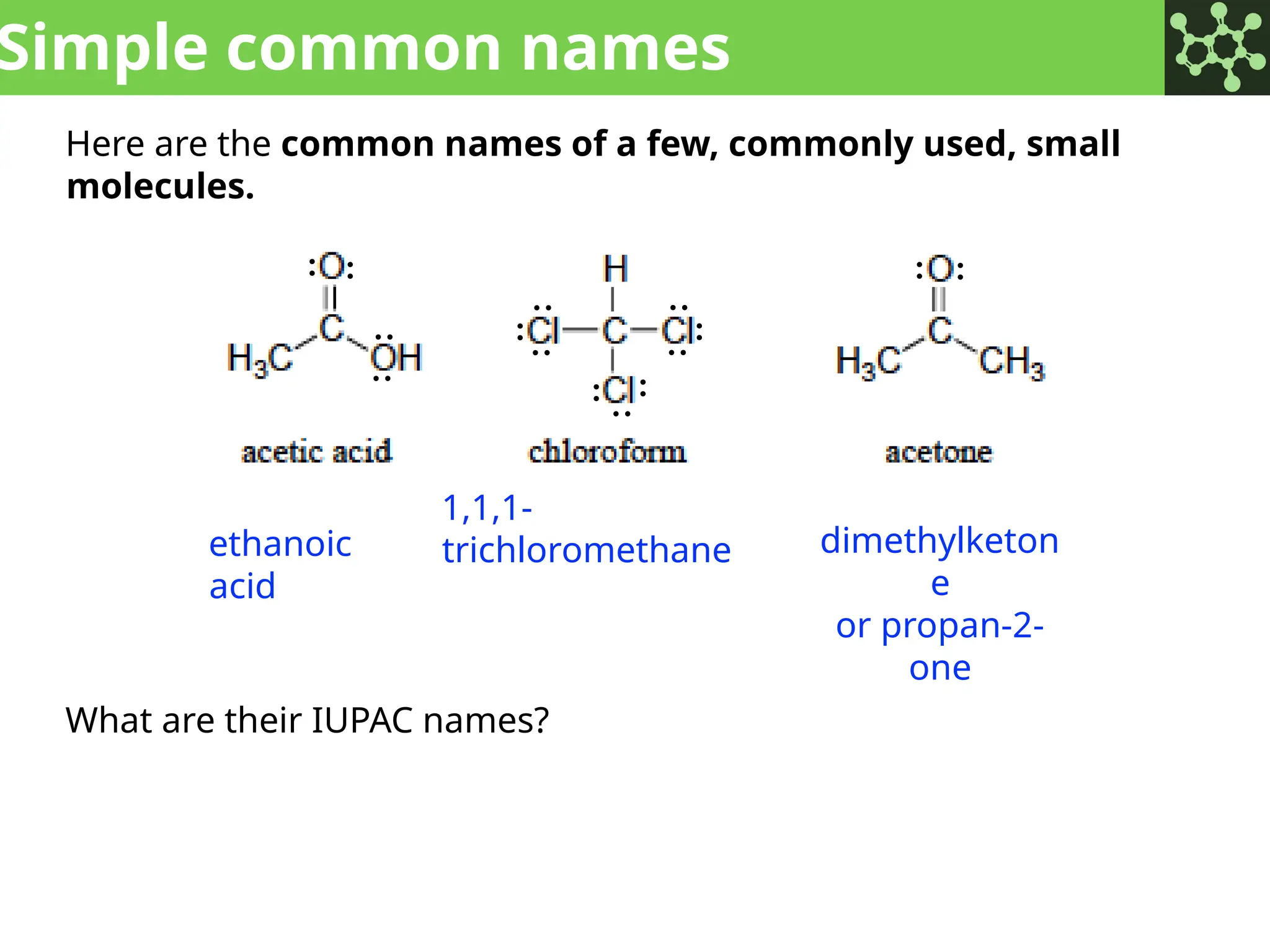
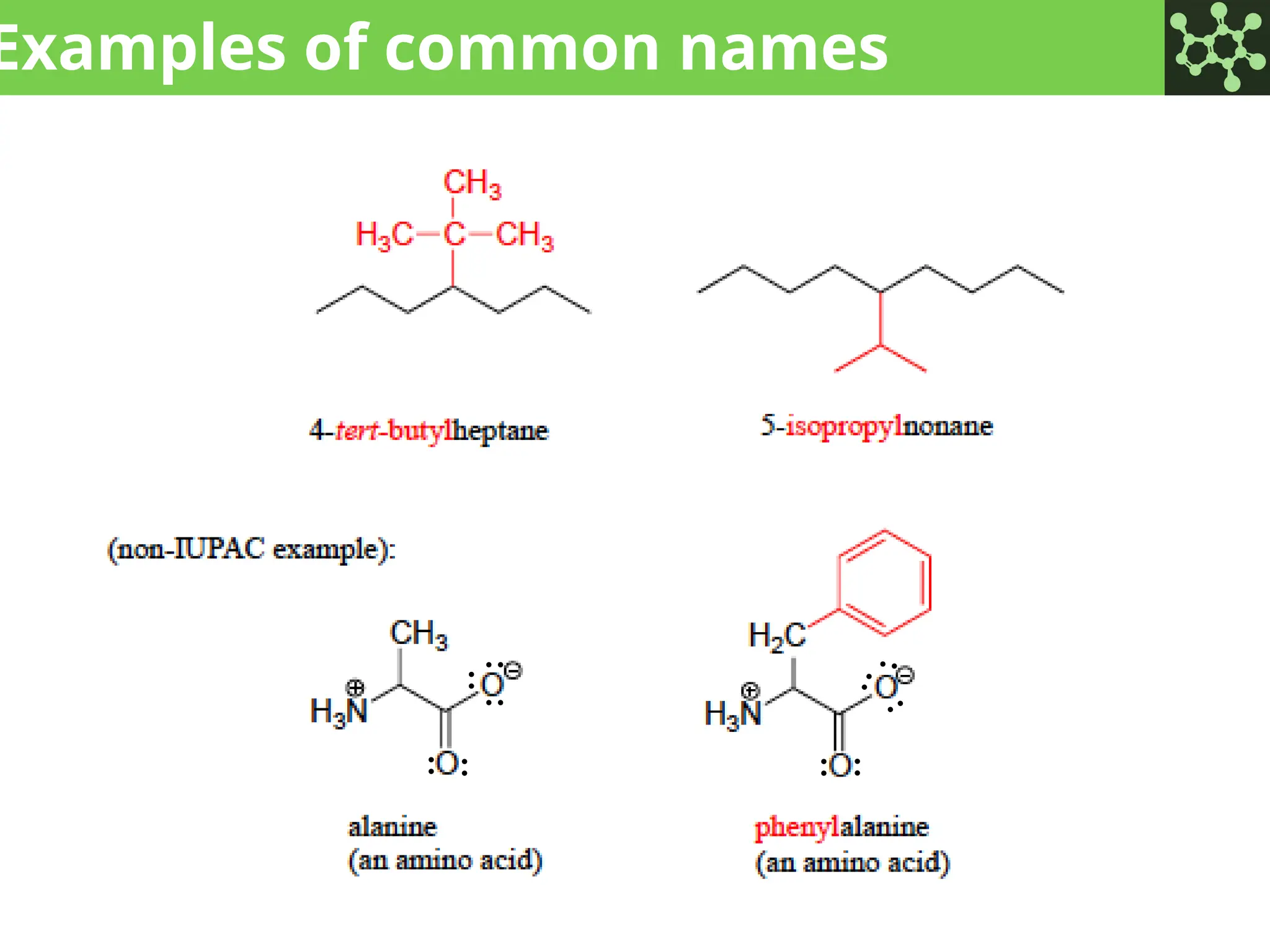
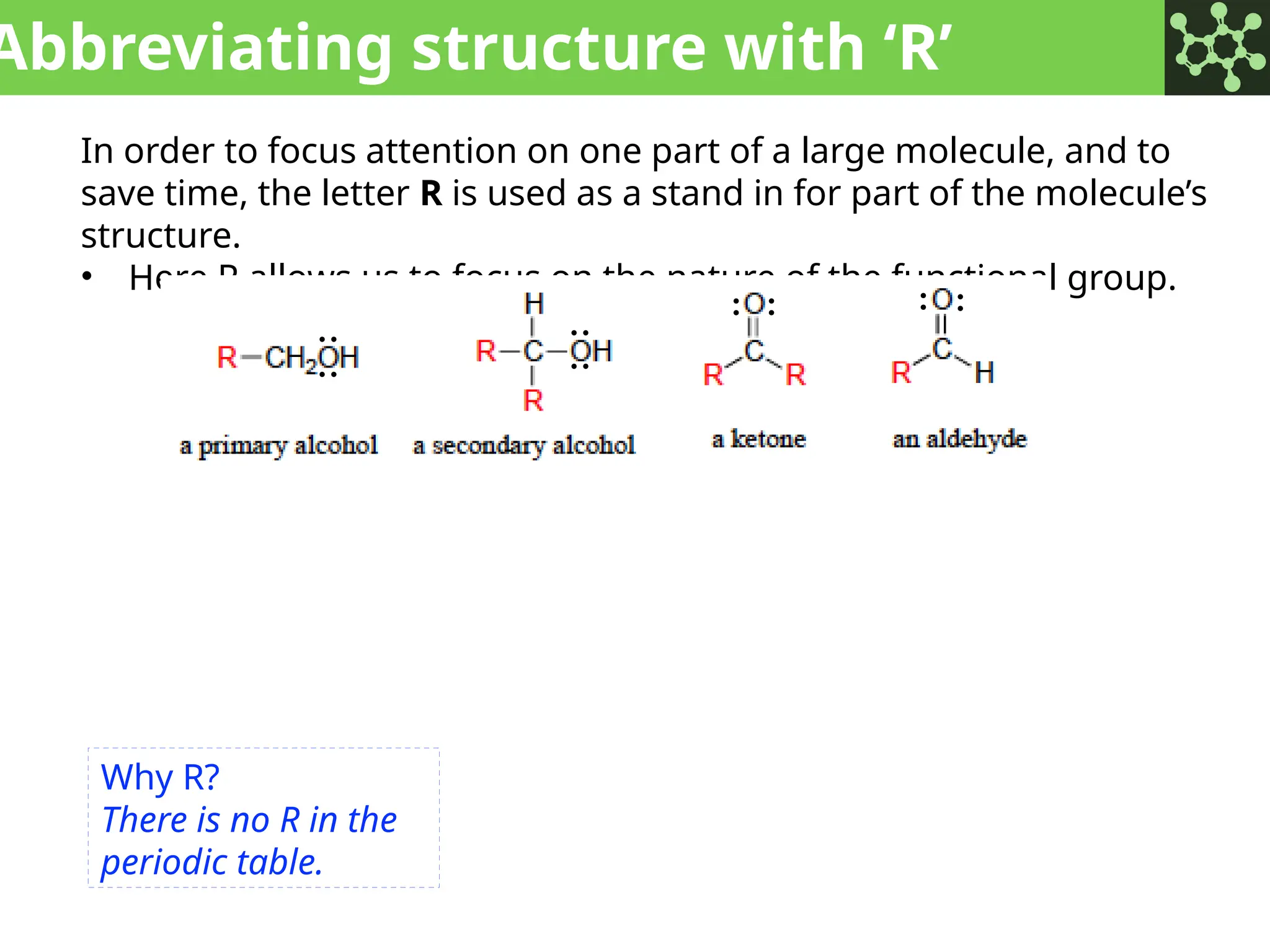
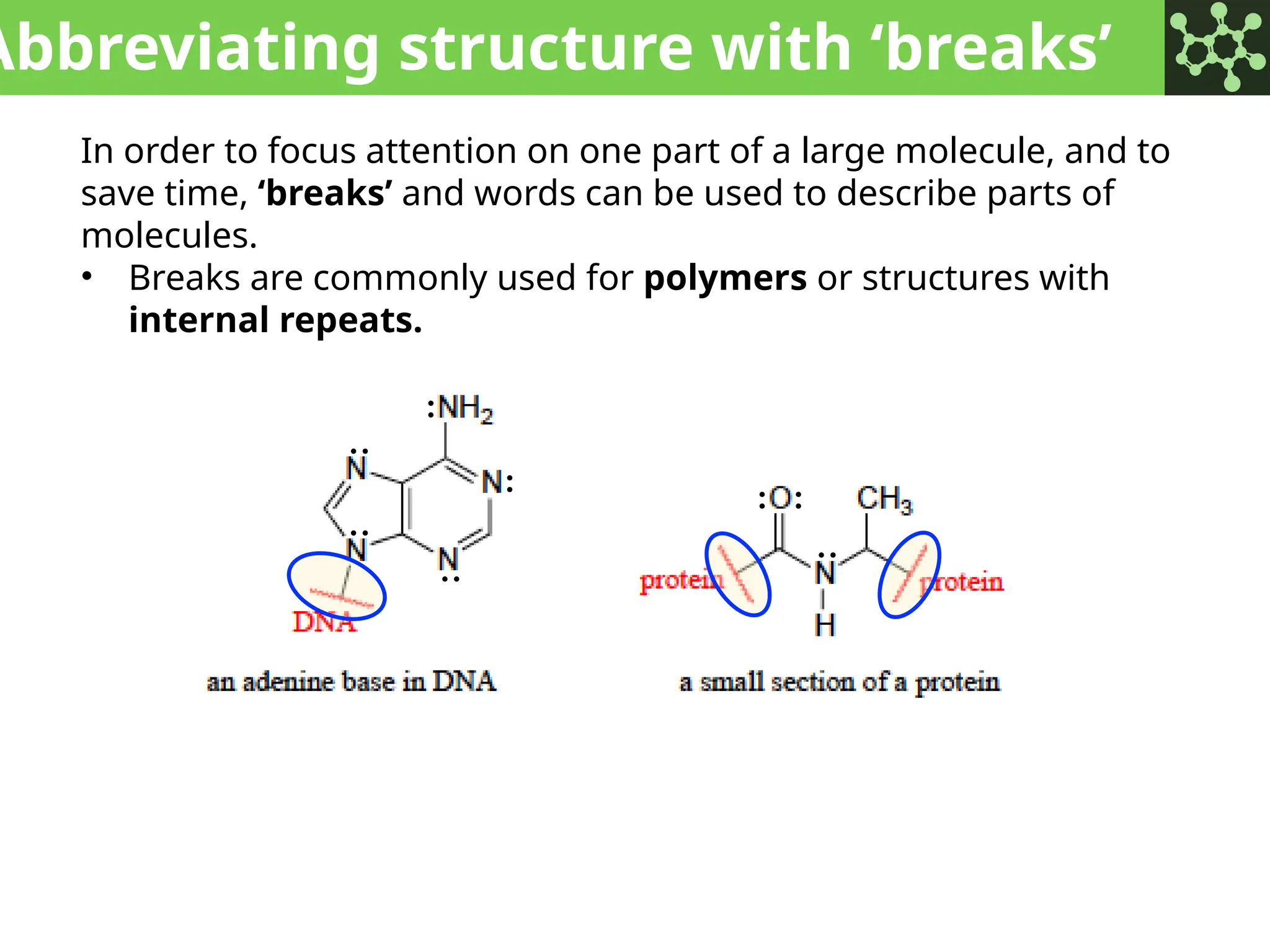
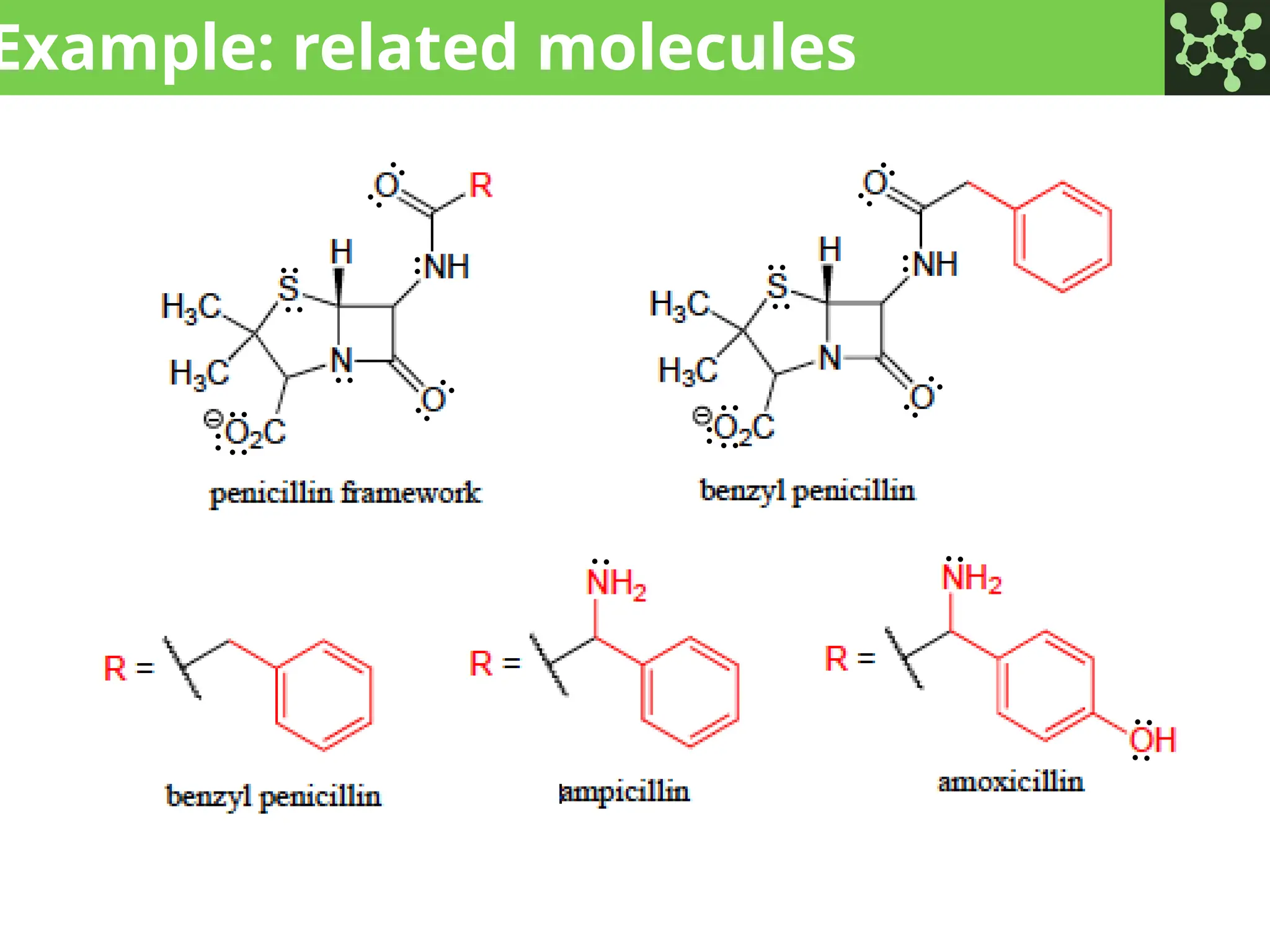
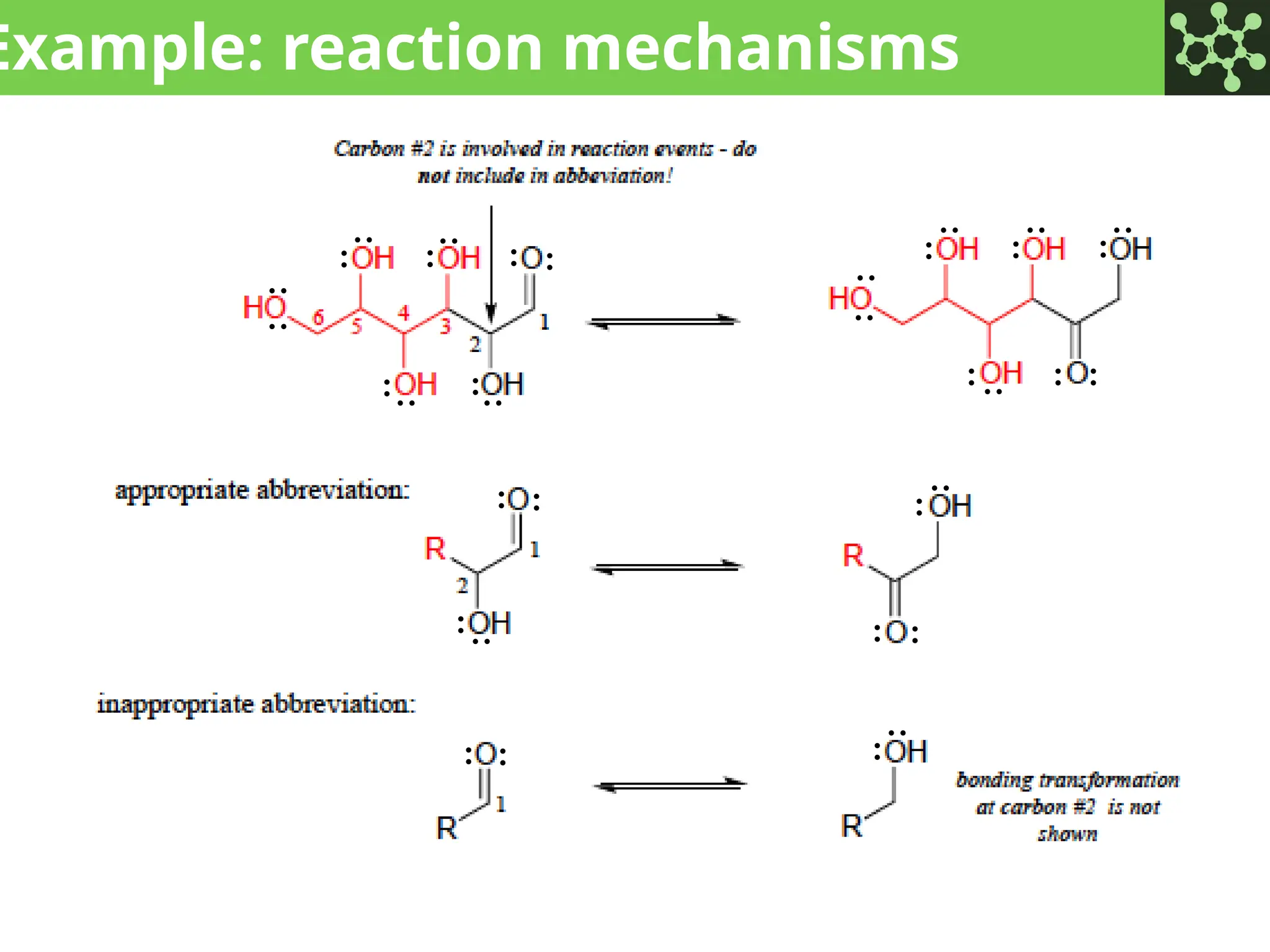
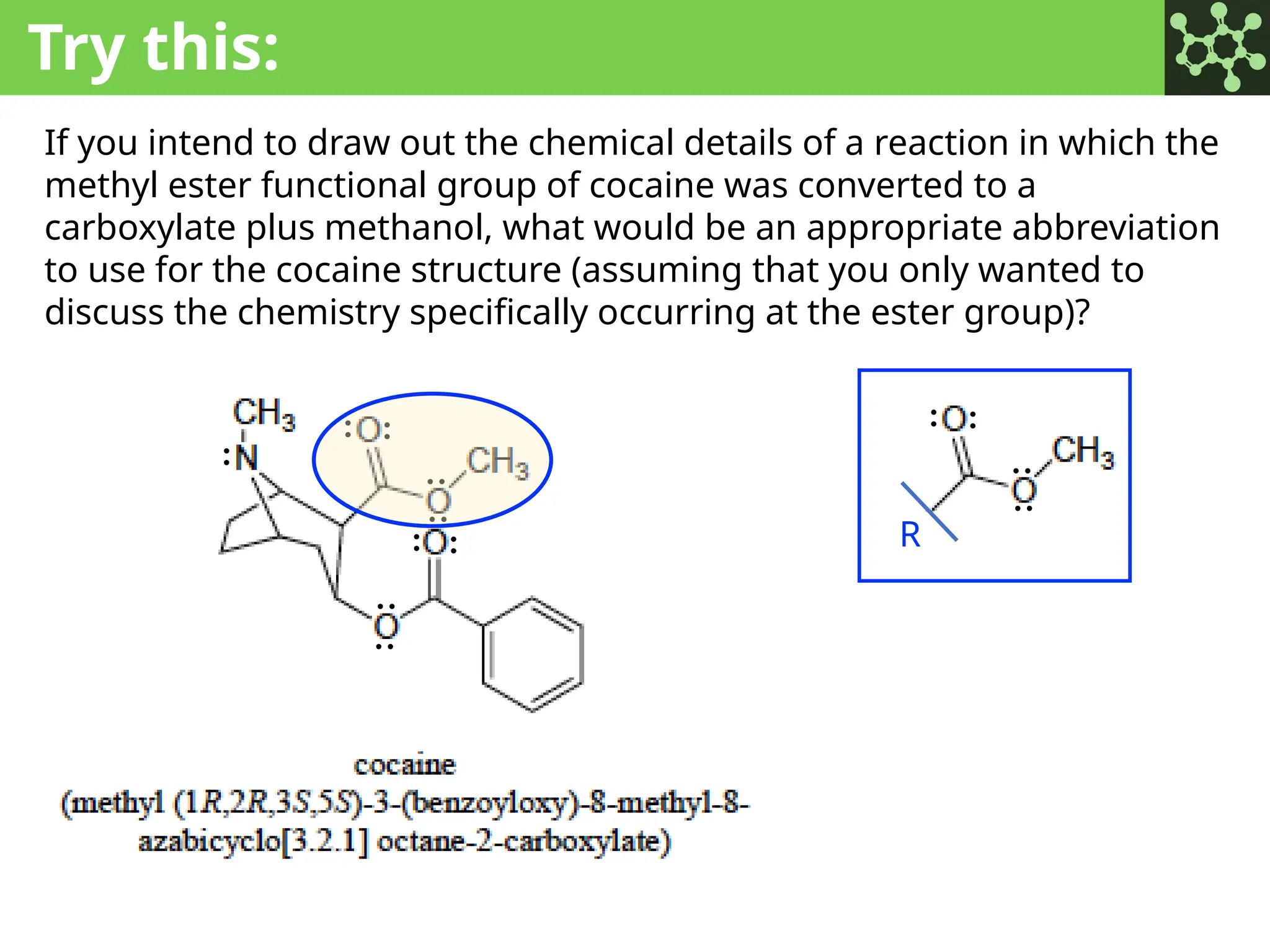
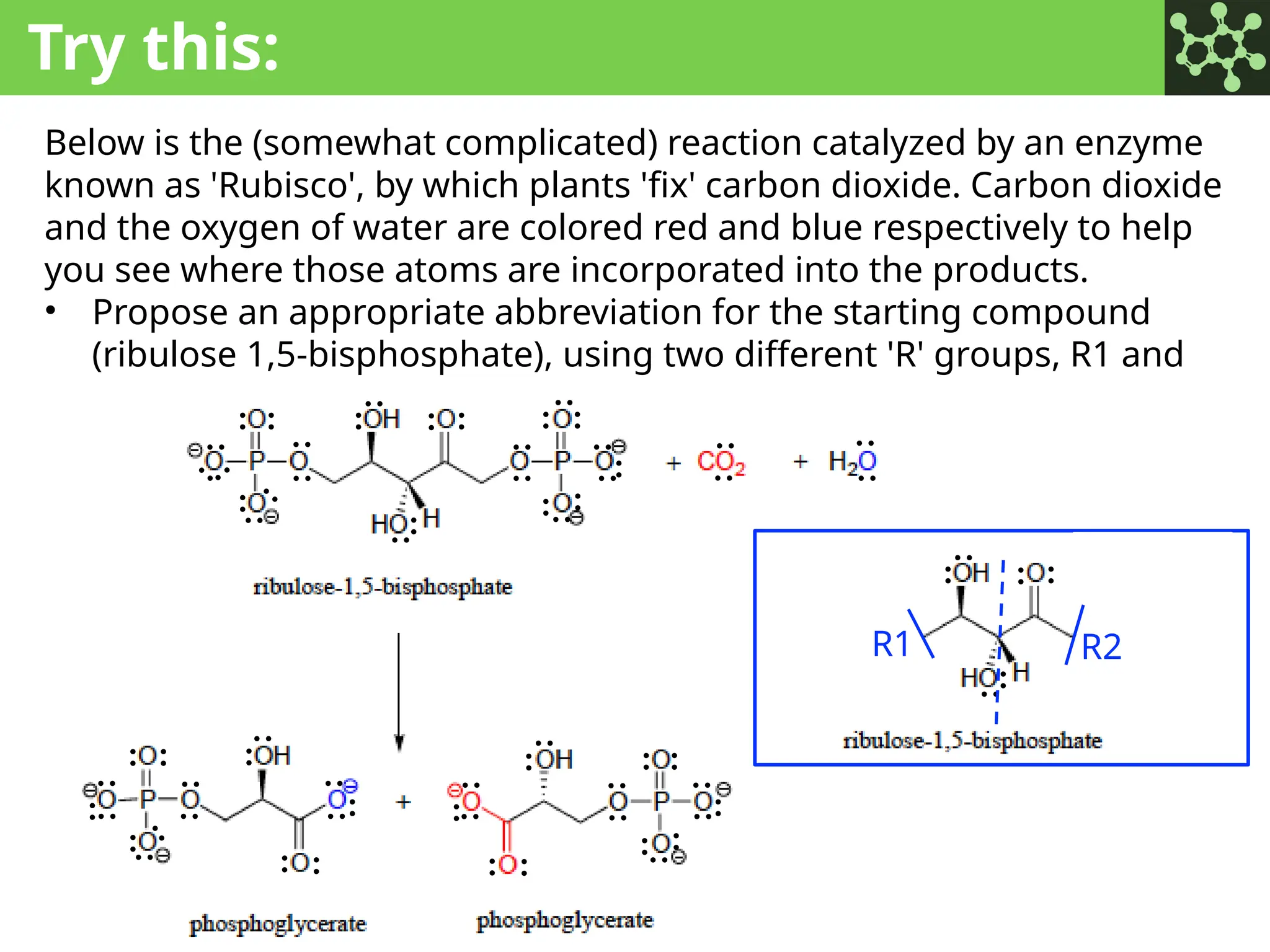
The document discusses the principles of organic chemistry, covering topics like organic structure, functional groups, and nomenclature. It emphasizes the importance of functional groups in determining chemical reactivity and explains the IUPAC naming conventions for various organic compounds, including alkanes, alkenes, and cyclic compounds. Additionally, it introduces methods for abbreviating complex molecular structures to facilitate communication in chemical discussions.