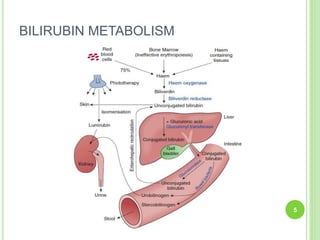
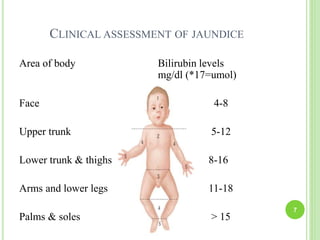
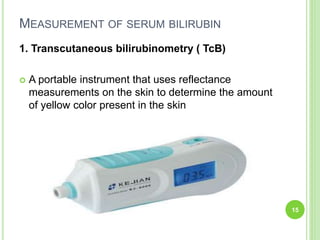
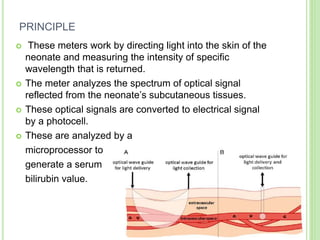
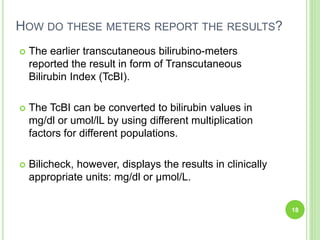
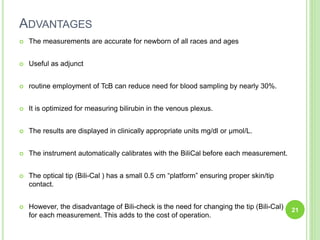
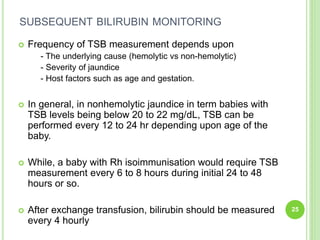
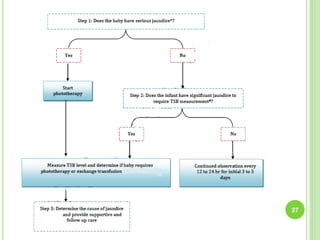
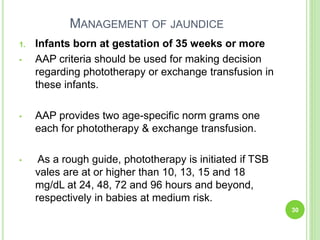
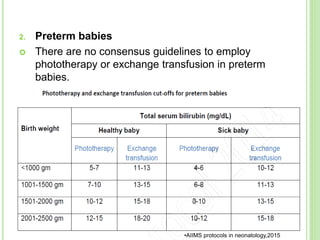
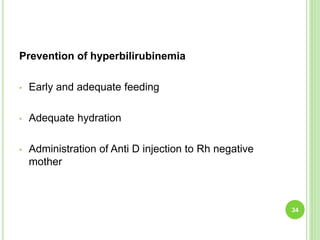
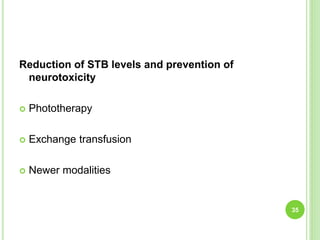
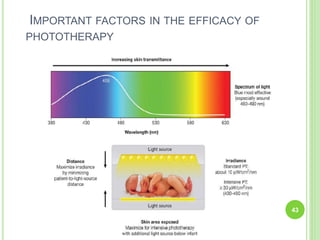
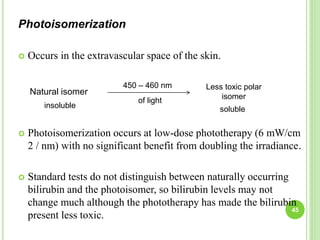
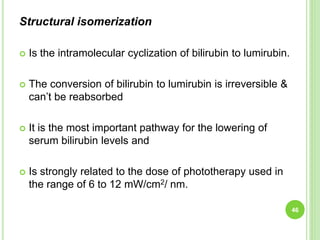
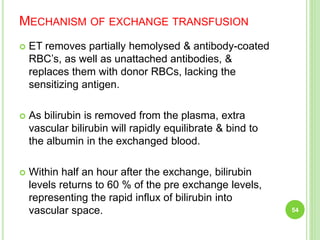
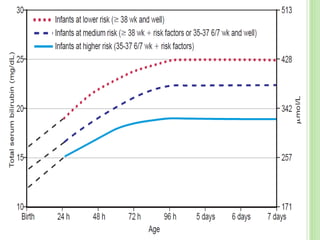
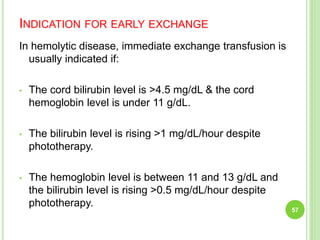
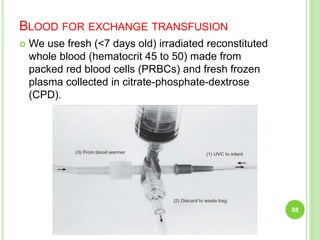
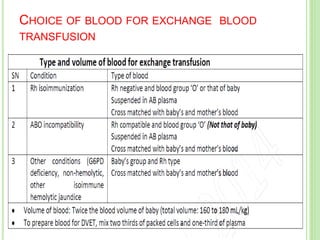
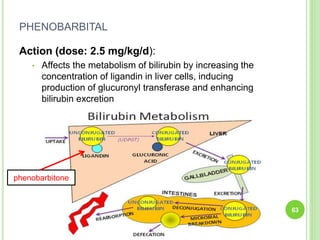
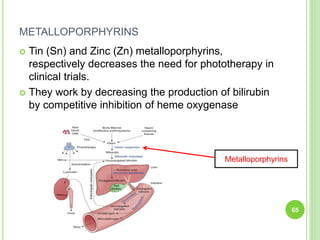
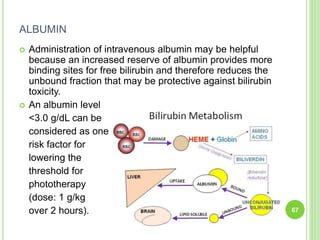
This document discusses current concepts in neonatal hyperbilirubinemia. It begins by describing bilirubin metabolism and the causes of hyperbilirubinemia. It then discusses the clinical assessment and diagnostic workup of jaundiced newborns. The main treatment options for hyperbilirubinemia are phototherapy and exchange transfusion. Phototherapy works by converting bilirubin into less toxic forms through photoisomerization, structural isomerization, and photo-oxidation reactions. Factors like light intensity and wavelength affect the efficacy of phototherapy.