The document describes 4 experiments related to analyzing proteins:
1) The ninhydrin reaction identifies amino acids by producing a purple color.
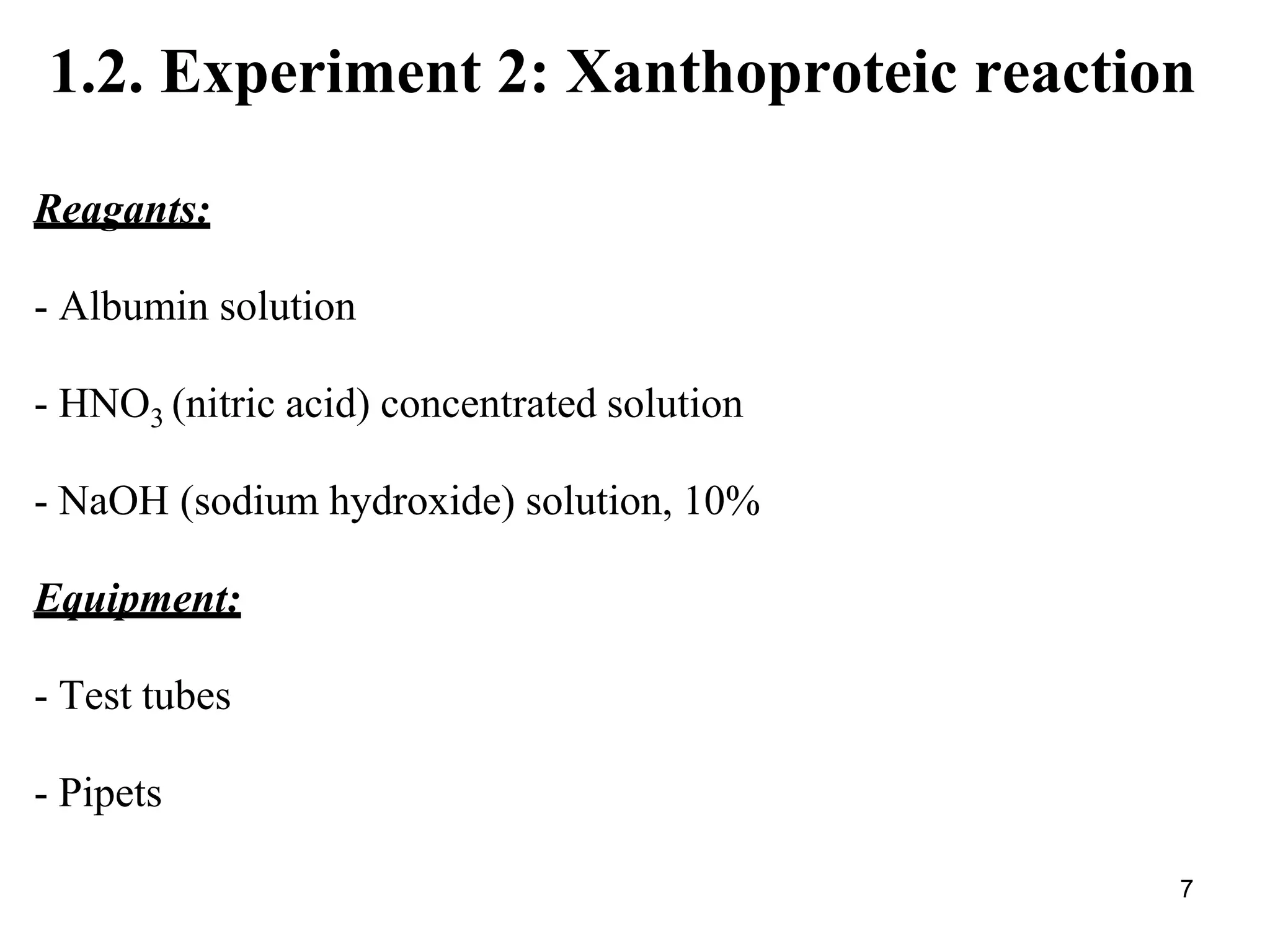
2) The xanthoproteic reaction detects aromatic amino acids in proteins by producing a yellow then orange color with nitric acid and sodium hydroxide.
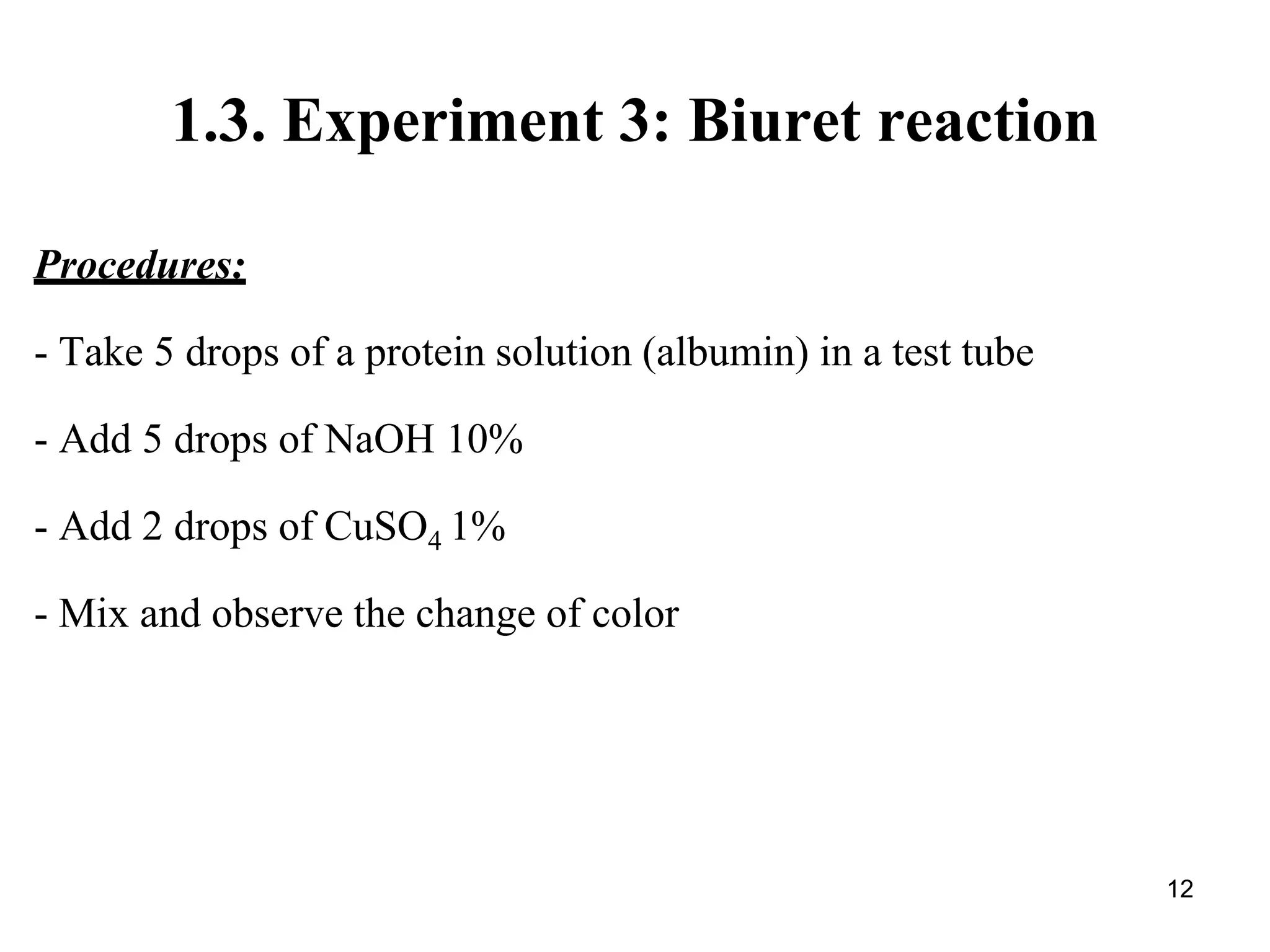
3) The biuret reaction identifies proteins using copper sulfate which complexes with peptide bonds to form a blue-violet color in alkaline conditions.
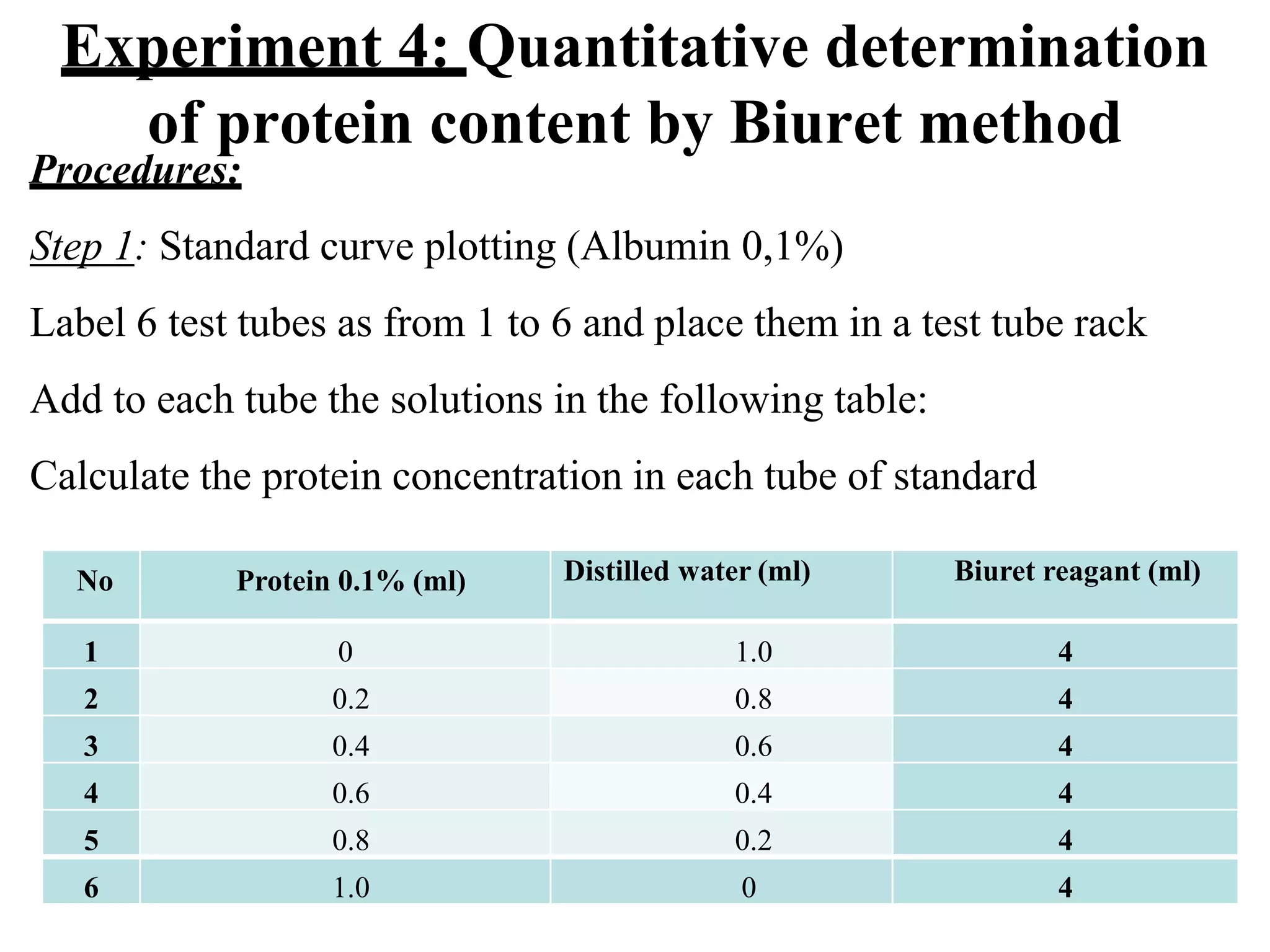
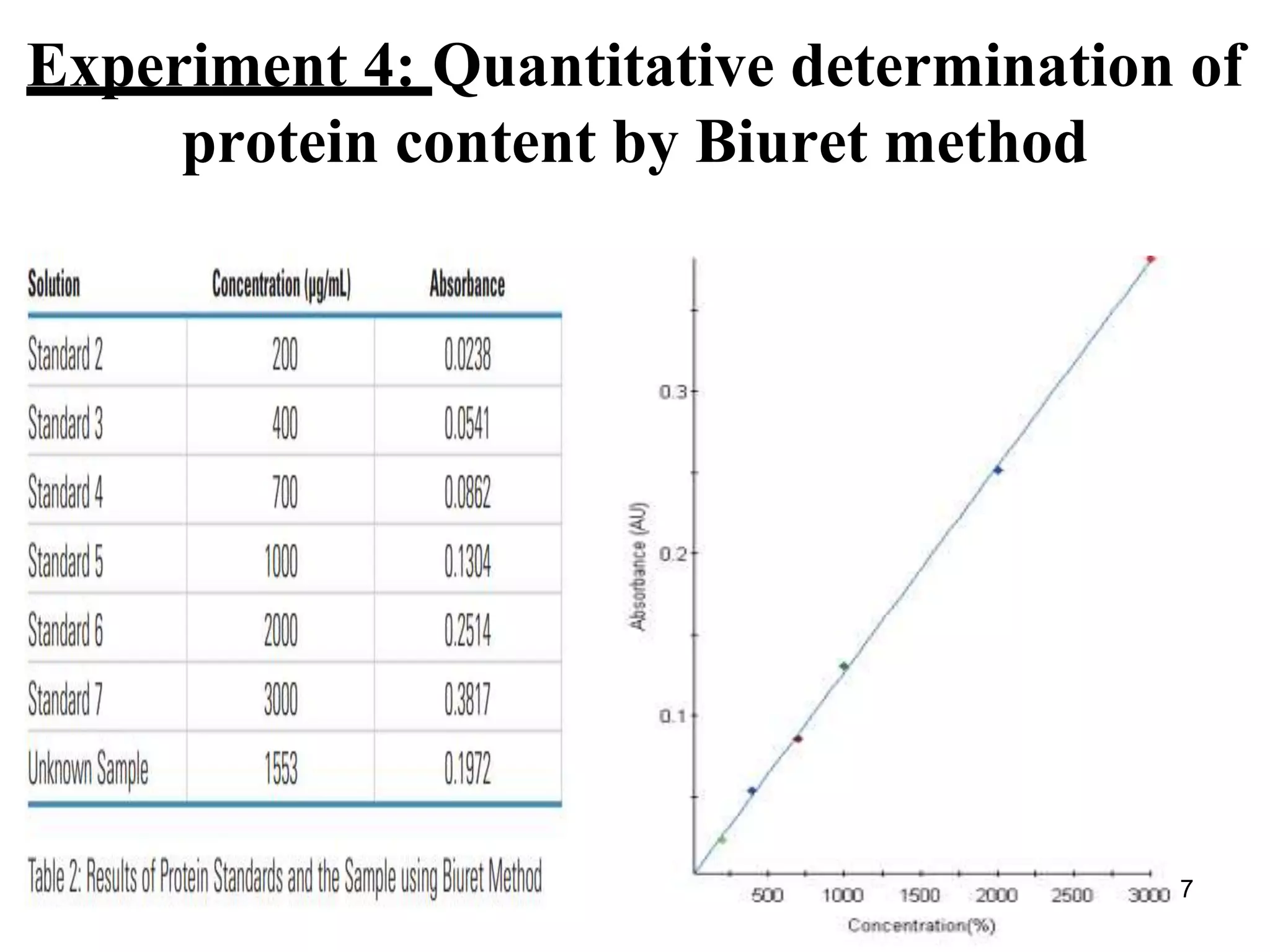
4) The biuret method is used to quantitatively determine protein content by measuring absorbance of biuret complexes at 540nm and comparing to a standard curve.