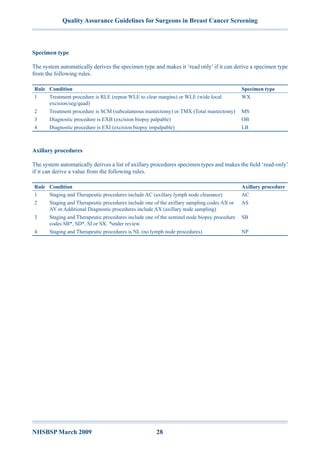
The document outlines quality assurance guidelines for surgeons involved in breast cancer screening within the NHS, aimed at ensuring high standards of care and diagnosis. It emphasizes the importance of assessment, diagnosis, and treatment processes, as well as adherence to waiting time targets to minimize patient anxiety. Additionally, the guidelines support collaboration within multidisciplinary teams to enhance the management of screen-detected breast cancer cases.