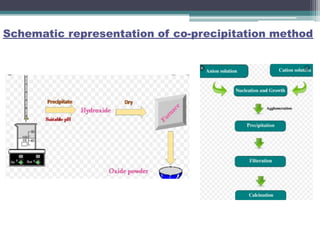
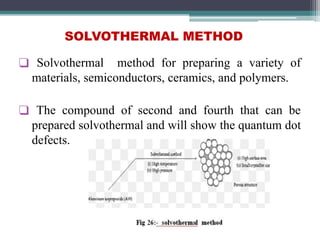
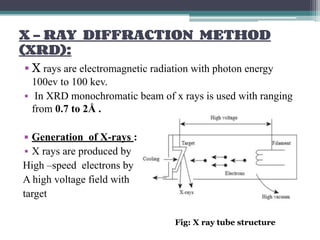
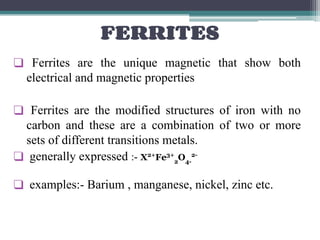
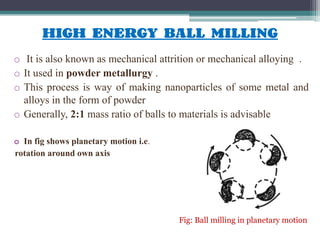
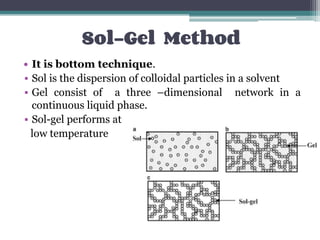
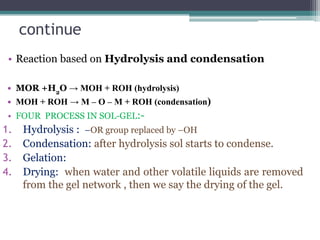
This document discusses nanoscience and nanotechnology, with a focus on ferrites. It defines nanoscience and describes nanomaterials, classifying them based on dimensionality. Ferrites are introduced as magnetic materials made of iron and other transition metals. Common ferrite types include spinel, garnet, and hexa ferrites. Synthesis techniques for ferrite nanoparticles are covered, including high energy ball milling, sol-gel, hydrothermal, and co-precipitation methods. Characterization techniques like XRD and FTIR are also summarized.



![NANOMATERIALS
❖ Nanomaterials is the set of substances , in
which at least one dimension is less than
100nm.Examples: fullerene, carbon nanotubes,
and graphene etc.
Fig 1: size comparsions of objects and nanomaterials [kumar,2016]](https://image.slidesharecdn.com/ferritesppt-220728081340-bdba299b/85/ferrites-ppt-ppt-4-320.jpg)
![CLASSIFICATION OF
NANOMATERIALS
On the based on dimensionality:-
• 0D NMs :- clusters and particles
• 1D NMs :- nanotubes and nanowires
• 2D NMs :- Nanoplates and layers
• 3D NMs :- Nanodiamond
Fig 2 :-(a)fullerene; (b)quantumdot;(c)metalcluster;
(d)carbonnanotube;(e)metaloxidenanotube;
(f)graphene; (g) metal oxide nanobelts;
(h) nanodiamond [kumar ,2016]](https://image.slidesharecdn.com/ferritesppt-220728081340-bdba299b/85/ferrites-ppt-ppt-5-320.jpg)
![CLASSIFICATION OF FERRITES
Based on magnetic materials Based on structures
1. Soft ferrites (MO.Fe2
O3.
)
• Soft ferrites are the ferromagnetic
material with cubic crystal structure.
• Soft ferrites are easily magnetized
and demagnetized. Example: NiZn
2 Hard ferrites
• Hard ferrites are composed of
iron and barium oxides.
• This types of ferrites are high
coercivity and high remanece
after magnetization.
Example: barrium ferrite(BaFe12
O19
)
1. Spinel ferrites(M2
Fe2
3+
O4
)
• Normal spinel ferrites :(M)A
[Fe2
]B
O4
• Inverse spinel ferrites (Fe)A
[MFe]B
O4
• Mixed spinel ferrites
(M1-x
Fe)[Fe2-x
Mx
]O4
2 Garnetferrites (R3
3+
Fe5
3+
O12
)
3 Ortho Ferrite
4 Hexa Ferrite : MFe12
O19](https://image.slidesharecdn.com/ferritesppt-220728081340-bdba299b/85/ferrites-ppt-ppt-7-320.jpg)




![HYDROTHERMAL
METHOD
• The term ‘hydrothermal’ comes from the Greek word
“hydrous”which means water and “thermal” means
heat
• Hydrothermal process occurs above the (200°C)
temperature, and pressure more than 100 bars.
• Al(OH)3
+ OH-
→[AlO(OH)2
)-
+ H2
O]
• AlOOH+OH-
→ [ AlO(OH)2
]](https://image.slidesharecdn.com/ferritesppt-220728081340-bdba299b/85/ferrites-ppt-ppt-15-320.jpg)