The document is a seminar presentation on nano-priming, a technique involving the application of nanoparticles to improve seed germination and plant growth in medicinal and aromatic plants. It covers the history, various priming methods, and details the effects of biologically synthesized nanoparticles on seed germination, seedling growth, and physiological changes in plants. Several case studies are included, illustrating the successful application of nano-priming with different nanoparticles across various plant species.










![Table 1. Influence of nano-priming plant growth and metabolism
Effect on plant physiology Changes in chemical
constituents
Molecular changes in
plant
Growth & biomass,
Photosynthesis [Chlorophyll]
Photosynthetic quantum efficiency,
Gas exchange traits,
Carotenoids,
Cellular electron exchange,
Soluble proteins,
Relative water content,
Antioxidant enzymes [POX, SOD,
CAT & APX]
Gibberellic acid
IAA
ABA:GA
α-amylase
Soluble sugar
Aquaporins
Lipids
DNA content
DNA repair
Cell wall extension (NtLRX1)
Cell division (CycB)
Aquaporin (PIP1, PIP2, NIP1, TIP3
& TIP4)
Phynylalanine ammonia lysase
(PAL1)
Anthocyanin synthase 1 (ANS1) and
Anthocyanin pigment 1 (PAP1)
11/48
Nile et al., 2022](https://image.slidesharecdn.com/nano-priming-anemergingtechnologyinmedicinalandaromaticplants-240904113955-fa825d3f/75/Nano-priming-An-Emerging-technology-in-Medicinal-and-Aromatic-Plants-pptx-11-2048.jpg)





















![Material and Methods
• Preparation of chitosan nanoparticles
• Seeds (Coated and uncoated) were imbibed in each concentration of chitosan
nanoparticles (CsNPs) (0.1, 0.5, 1 mg/ ml) for 4, 8 and 12 h
• Germination bioassay – Petri dish assay
• Growth experiment – In plastic pots
• Growth Parameters – Germination percentage, Gemination velocity, Speed
of germination, Germination energy, Germination index, Mean germination
time and Seedling vigour index
• Determination of plant biomass and water content
• Determination of phytochemicals [Alkaloid] – GC-MS
• Antioxidant activity
Allam et al., 2024 33/48](https://image.slidesharecdn.com/nano-priming-anemergingtechnologyinmedicinalandaromaticplants-240904113955-fa825d3f/75/Nano-priming-An-Emerging-technology-in-Medicinal-and-Aromatic-Plants-pptx-33-2048.jpg)







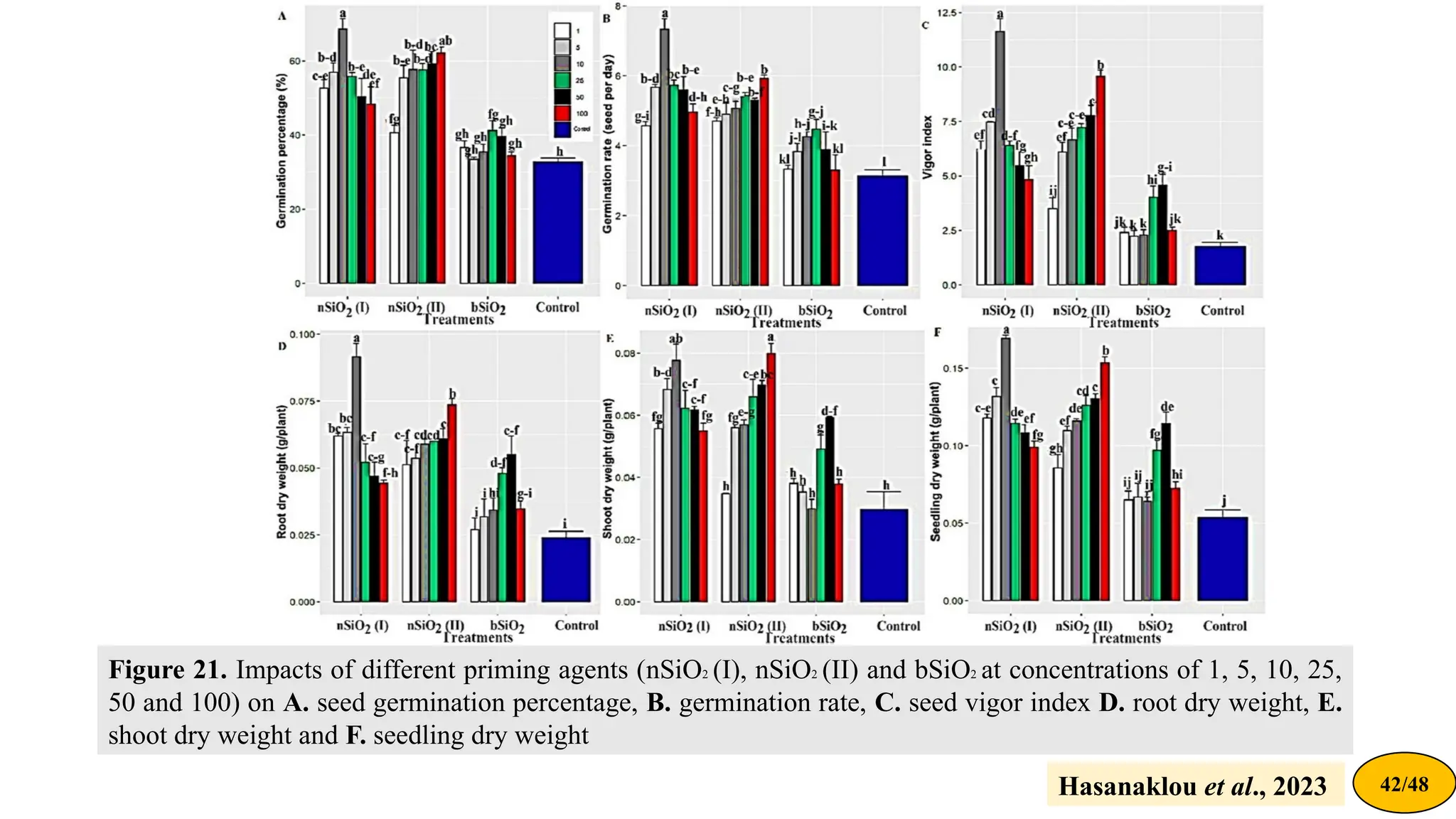
![Material and methods
• Synthesis of nanoparticles (nSiO2 (I) and nSiO2 (II) showed
the silica NPs synthesized at 85 °C and 25 °C, respectively)
• Seed treatment with different concentrations (0, 1, 5, 10, 25,
50, and 100 ppm) of nSiO2 (I), nSiO2 (II), and commercial
bulk SiO2 (bSiO2)
• Germination percentage, germination rate & seed vigour
• Starch and sucrose determination
• Antioxidant enzymes activity [CAT & POX]
Hasanaklou et al., 2023 41/48](https://image.slidesharecdn.com/nano-priming-anemergingtechnologyinmedicinalandaromaticplants-240904113955-fa825d3f/75/Nano-priming-An-Emerging-technology-in-Medicinal-and-Aromatic-Plants-pptx-41-2048.jpg)