Stevens-Johnson syndrome is an immune-mediated condition that causes blistering and lesions of the skin and mucous membranes. It can be caused by certain infections, medications, and other factors. Acetaminophen use increases the risk of developing Stevens-Johnson syndrome. Signs include rashes, blisters, and lesions affecting the eyes, mouth, genitals or other mucous membranes. Treatment focuses on supportive care, stopping any triggering medications, and managing symptoms.

![1- Stevens-Johnson Syndrome
Stevens-Johnson syndrome is an immune-complex–mediated hypersensitivity complex
that typically involves the skin and the mucous membranes. Although several
classification schemes have been reported, the simplest classification breaks the disease
down as follows[1] :
Stevens-Johnson syndrome: A minor form of toxic epidermal necrolysis, with less
than 10% body surface area (BSA) detachment
Overlapping Stevens-Johnson syndrome/toxic epidermal necrolysis: Detachment
of 10-30% of the BSA
Toxic epidermal necrolysis: Detachment of more than 30% of the BSA
Essential update: Acetaminophen raises risk of Stevens-Johnson syndrome
In August 2013, the FDA issued a safety announcement advising that anyone who
develops a rash, blister, or some other skin reaction while taking acetaminophen should
stop using the drug and seek medical care immediately.[2, 3] The drug poses a risk for the
following 3 rare but potentially fatal skin disorders:
Stevens-Johnson syndrome
Toxic epidermal necrolysis
Acute generalized exanthematous pustulosis
The FDA warning was based on a review of medical literature and cases of adverse
reactions reported to the FDA Adverse Event Reporting System (FAERS) database.[3]
Signs and symptoms
Typical prodromal symptoms of Stevens-Johnson syndrome are as follows:
Cough productive of a thick, purulent sputum
Headache
Malaise
Arthralgia
Patients may complain of a burning rash that begins symmetrically on the face and the
upper part of the torso. The cutaneous lesions are characterized as follows:
The rash can begin as macules that develop into papules, vesicles, bullae,
urticarial plaques, or confluent erythema](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-2-320.jpg)


![A patient with severe eye involvement associated with Stevens-Johnson syndrome. Note
corneal neovascularization and conjunctivalization of the ocular surface.
Background
Stevens-Johnson syndrome (SJS) is an immune-complex–mediated hypersensitivity
complex that typically involves the skin and the mucous membranes. While minor
presentations may occur, significant involvement of oral, nasal, eye, vaginal, urethral,
gastrointestinal, and lower respiratory tract mucous membranes may develop in the
course of the illness. GI and respiratory involvement may progress to necrosis. Stevens-
Johnson syndrome is a serious systemic disorder with the potential for severe morbidity
and even death.
The syndrome was first described in 1922, when the American pediatricians Albert
Mason Stevens and Frank Chambliss Johnson reported the cases of 2 boys aged 7 and 8
years with "an extraordinary, generalized eruption with continued fever, inflamed buccal
mucosa, and severe purulent conjunctivitis." Both cases had been misdiagnosed by
primary care physicians as hemorrhagic measles.
Erythema multiforme (EM), originally described by von Hebra in 1866, was part of the
differential diagnosis in both cases but was excluded because of the "character of skin
lesions, the lack of subjective symptoms, the prolonged high fever, and the terminal
heavy crusting." Despite the presence of leukopenia in both cases, Stevens and Johnson
in their initial report suspected an infectious disease of unknown etiology as the cause.
In 1950, Thomas divided EM into 2 categories: erythema multiforme minor (von Hebra)
and erythema multiforme major (EMM). Since 1983, erythema multiforme major and
Stevens-Johnson syndrome had been considered synonymous.
In the 1990s, however, Bastuji and Roujeau each proposed that erythema multiforme
major and Stevens-Johnson syndrome are 2 distinct disorders.[4] They suggested that the
denomination of erythema multiforme should be restricted to patients with typical targets
or raised edematous papules, with or without mucosal involvement. This clinical picture
is in accordance with the original description by von Hebra.
Bastuji and Roujeau further proposed that the denomination of Stevens-Johnson
syndrome should be used for a syndrome characterized by mucous membrane erosions
and widespread small blisters that arise on erythematous or purpuric maculae that are
different from classic targets.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-5-320.jpg)
![According to this clinical classification, erythema multiforme major and Stevens-Johnson
syndrome could be 2 distinct disorders with similar mucosal erosions, but different
patterns of cutaneous lesions. This hypothesis is supported further by a strong correlation
between clinical classification and the probable cause.
Conversely, several investigators propose that Stevens-Johnson syndrome and toxic
epidermal necrolysis (TEN) represent the same disease at different levels of severity. A
unifying classification of "acute disseminated epidermal necrosis" or "exanthematic
necrolysis" has been suggested.
Although several classification schemes have been reported, the simplest breaks the
disease down as follows[1] :
Stevens-Johnson syndrome - A "minor form of TEN," with less than 10% body
surface area (BSA) detachment
Overlapping Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) -
Detachment of 10-30% BSA
Toxic epidermal necrolysis - Detachment of more than 30% BSA
An argument against this unifying concept was that HSV infection had been described as
a frequent cause of Stevens-Johnson syndrome/erythema multiforme major but not of
toxic epidermal necrolysis. However, reports showed that HSV infection has not been
related to Stevens-Johnson syndrome, and suggested that clinical manifestations and
pathology results support the linking of Stevens-Johnson syndrome and toxic epidermal
necrolysis, and their differentiation from erythema multiforme.
Various etiologic factors (eg, infection, drugs, malignancies) have been implicated as
causes of Stevens-Johnson syndrome. However, as many as half of cases are idiopathic.
There is strong evidence for a genetic predisposition to Stevens-Johnson syndrome
provoked by certain drugs.
There are no specific laboratory studies (other than biopsy) that can definitively establish
the diagnosis of Stevens-Johnson syndrome .No specific treatment of Stevens-Johnson
syndrome is noted; most patients are treated symptomatically. In principle, the
symptomatic treatment of patients with Stevens-Johnson syndrome does not differ from
the treatment of patients with extensive burns. Withdrawal of the suspected offending
agent is critically important. Immunomodulatory treatment is controversial.
Pathophysiology
An idiosyncratic, delayed hypersensitivity reaction has been implicated in the
pathophysiology of Stevens-Johnson syndrome. Certain population groups appear more
susceptible to develop Stevens-Johnson syndrome than the general population. Slow
acetylators, patients who are immunocompromised (especially those infected with HIV[5,
6] ), and patients with brain tumors undergoing radiotherapy with concomitant
antiepileptics are among those at most risk.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-6-320.jpg)
![Slow acetylators are people whose liver cannot completely detoxify reactive drug
metabolites. For example, patients with sulfonamide-induced toxic epidermal necrolysis
have been shown to have a slow acetylator genotype that results in increased production
of sulfonamide hydroxylamine via the P-450 pathway. These drug metabolites may have
direct toxic effects or may act as haptens that interact with host tissues, rendering them
antigenic.[7, 8]
Antigen presentation and production of tumor necrosis factor (TNF)–alpha by the local
tissue dendrocytes results in the recruitment and augmentation of T-lymphocyte
proliferation and enhances the cytotoxicity of the other immune effector cells.[9] A "killer
effector molecule" has been identified that may play a role in the activation of cytotoxic
lymphocytes.[10] The activated CD8+ lymphocytes, in turn, can induce epidermal cell
apoptosis via several mechanisms, which include the release of granzyme B and perforin.
In 1997, Inachi et al demonstrated perforin-mediated apoptosis in patients with Stevens-
Johnson syndrome.[11] Perforin, a pore-making monomeric granule released from natural
killer cells and cytotoxic T lymphocytes, kills target cells by forming polymers and
tubular structures not unlike the membrane attack complex of the complement system.
Apoptosis of keratinocytes can also take place as a result of ligation of their surface death
receptors with the appropriate molecules. Those can trigger the activation of the caspase
system, leading to DNA disorganization and cell death.[12]
Apoptosis of keratinocytes can be mediated via direct interaction between the cell-death
receptor Fas and its ligand. Both can be present on the surfaces of the keratinocytes.
Alternatively, activated T-cells can release soluble Fas ligand and interferon-gamma,
which induces Fas expression by keratinocytes.[1] Researchers have found increased levels
of soluble Fas ligand in the sera of patients with SJS/TEN before skin detachment or
onset of mucosal lesions.[13]
The death of keratinocytes causes separation of the epidermis from the dermis. Once
apoptosis ensues, the dying cells provoke recruitment of more chemokines. This can
perpetuate the inflammatory process, which leads to extensive epidermal necrolysis.[14]
Higher doses and rapid introduction of allopurinol[15] and lamotrigine[16] may also increase
the risk of developing SJS/TEN. Risk is lessened by starting these at the low doses and
titrating gradually.[17]
There is evidence that systemic lupus is a risk factor as well.[18]
Etiology
Various etiologic factors have been implicated as causes of Stevens-Johnson syndrome.
Drugs most commonly are blamed. The 4 etiologic categories are as follows:
Infectious
Drug-induced
Malignancy-related
Idiopathic](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-7-320.jpg)
![Stevens-Johnson syndrome is idiopathic in 25-50% of cases. Drugs and malignancies are
most often implicated as the etiology in adults and elderly persons. Pediatric cases are
related more often to infections.
1. Infectious causes
Viral diseases that have been reported to cause Stevens-Johnson syndrome include the
following:
Herpes simplex virus (possibly; remains a debated issue)
AIDS
Coxsackie viral infections
Influenza
Hepatitis
Mumps
In children, Epstein-Barr virus and enteroviruses have been identified. More than half of
the patients with Stevens-Johnson syndrome report a recent upper respiratory tract
infection.
Bacterial etiologies include the following:
Group A beta-hemolytic streptococci
Diphtheria
Brucellosis
Lymphogranuloma venereum
Mycobacteria
Mycoplasma pneumoniae[19, 20]
Rickettsial infections
Tularemia
Typhoid
Possible fungal causes include coccidioidomycosis, dermatophytosis, and histoplasmosis.
Malaria and trichomoniasis have been reported as protozoal causes.
2. Drug-induced
Antibiotics are the most common cause of Stevens-Johnson syndrome, followed by
analgesics, cough and cold medication, NSAIDs, psychoepileptics, and antigout drugs.
Of antibiotics, penicillins and sulfa drugs are prominent; ciprofloxacin has also been
reported[21]
The following anticonvulsants have been implicated:
Phenytoin
Carbamazepine
oxcarbazepine (Trileptal)](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-8-320.jpg)
![ Valproic acid
Lamotrigine
Barbiturates
Mockenhapupt et al stressed that most anticonvulsant-induced SJS occurs in the first 60
days of use.[22]
Antiretroviral drugs implicated in Stevens-Johnson syndrome include nevirapine and
possibly other non-nucleoside reverse transcriptase inhibitors.[23] Indinavir has been
mentioned.
Stevens-Johnson syndrome has also been reported in patients taking the following drugs:
Modafinil (Provigil)
Allopurinol[24]
Mirtazapine[25]
TNF-alpha antagonists (eg, infliximab, etanercept, adalimumab)[26]
Cocaine
Sertraline
Pantoprazole
Tramadol
3. Genetic factors
There is strong evidence for a genetic predisposition to severe cutaneous adverse drug
reactions such as Stevens-Johnson syndrome. Carriage of the following human leukocyte
antigens has been associated with increased risk:
HLA-B*1502
HLA-B*5801
HLA-B*44
HLA-A29
HLA-B12
HLA-DR7
HLA-A2
HLA-B*5801
HLA-A*0206
HLA-DQB1*0601
Certain of these HLA alleles are associated with an increased probability of developing
Stevens-Johnson syndrome upon exposure to specific drugs. The US Food and Drug
Administration (FDA) and Health Canada advise screening for HLA-B*1502 in patients
of southeastern Asian ethnicity before starting treatment with carbamazepine. (The risk is
much lower in other ethnic populations, making screening impractical in them). HLA-B*
5801 confers a risk of allopurinol-related reactions.[27] Pretreatment screening is not
readily available.[28]](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-9-320.jpg)
![Whites with HLA-B*44 appear to be more susceptible to develop Stevens-Johnson
syndrome. HLA-A29, HLA-B12, and HLA-DR7 are frequently associated with
sulfonamide-induced Stevens-Johnson syndrome, while HLA-A2 and HLA-B12 are often
encountered in Stevens-Johnson syndrome induced by nonsteroidal anti-inflammatory
drugs (NSAIDs).
HLA-A*0206 and HLA-DQB1*0601 allele have been shown to be was strongly
associated with Stevens-Johnson syndrome with ocular disease.[29, 30]
Nevertheless, whether the presence of those genes constitutes a predisposition to Stevens-
Johnson syndrome or whether those genes are in linkage disequilibrium with more
relevant adjacent genes is unknown.[31]
Epidemiology
Strom et al reviewed Medicaid billing data from 1980-1984 in Michigan, Minnesota, and
Florida to determine the incidence of Stevens-Johnson syndrome; the incidence rates
were 7.1, 2.6, and 6.8 cases per million population per year, respectively.[32]
Cases tend to have a propensity for the early spring and winter.
For overlapping SJS and TEN, oxicam NSAIDs (piroxicam, meloxicam, tenoxicam) and
sulfonamides are most commonly implicated in the United States and other western
nations.
SJS occurs with a worldwide distribution similar in etiology and occurrence to that in the
United States. However, a study from Germany reported only 1.1 cases per 1 million
person-years.
In contrast to the drugs most often implicated in western nations, allopurinol is the most
common offending agent in Southeast Asian nations, including Malaysia, Singapore,
Taiwan, and Hong Kong.
Race-, sex-, and age-related demographics
Stevens-Johnson syndrome has been described worldwide in all races, although it may be
more common in whites. Interestingly, disease is not limited to humans; cases have been
reported in dogs, cats, and monkeys.
The proportion of females has been estimated to be 33-62%. The largest series reports
39.9% of females in a group of 315 patients with Stevens-Johnson syndrome.
In a large cohort, the mean age of patients with Stevens-Johnson syndrome was 25 years.
In a smaller series, the mean age of patients with Stevens-Johnson syndrome was
reported as 47 years. However, cases have been reported in children as young as 3
months.
Prognosis](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-10-320.jpg)
![Individual lesions typically should heal within 1-2 weeks, unless secondary infection
occurs. Most patients recover without sequelae.
Mortality is determined primarily by the extent of skin sloughing. When body surface
area (BSA) sloughing is less than 10%, the mortality rate is approximately 1-5%.
However, when more than 30% BSA sloughing is present, the mortality rate is between
25% and 35%, and may be as high as 50% Bacteremia and sepsis appear to play a major
role in increased mortality.
The SCORTEN score (a severity-of-illness score for toxic epidermal necrolysis)
calculates the risk for death in both SJS and TEN on the basis of the following variables:
Age >40 years
Malignancy
Heart rate >120
Initial percentage of epidermal detachment >10%
Blood urea nitrogen (BUN) level >10 mmol/L
Serum glucose level >14 mmol/L
Bicarbonate level < 20 mmol/L
Each variable is assigned a value of 1 point. Mortality rates are as follows:
0-1 points, ≥3.2%
2 points, ≥12.1%
3 points, ≥35.3%
4 points, ≥58.3%
5 or more points, ≥90%
Other negative prognostic factors include persistent neutropenia (defined as neutropenia
lasting more than 5 days), hypoalbuminemia (usually < 2 g/dL), and persistent azotemia.
In a survival analysis of a cohort of patients with either Stevens-Johnson syndrome or
toxic epidermal necrolysis, Sekula et al found that the severity of the cutaneous reaction
causing either of these disorders was a risk factor for mortality, but only during the first
90 days following reaction onset.[35] The investigators also found that serious
comorbidities and age were risk factors for mortality after 90 days, but not beyond 1 year,
past reaction onset. Mortality among patients was 23% at 6 weeks and 34% at 1 year.
Although some patients rapidly progress to lose very large areas of the epidermis in a
matter of days, the process suddenly ceases in others and reepithelialization begins a few
days later. Predicting the course of disease in a given patient at the initial presentation is
not possible. Reepithelialization is usually complete within 3 weeks, but pressure and
mucosal areas may remain eroded and crusted for 2 weeks or longer.
Survivors of Stevens-Johnson syndrome may experience numerous long-term sequelae;
the most disabling are those of the eye. Cicatrization of conjunctival erosions may lead to
the following:](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-11-320.jpg)


![Several skin care approaches have been described. Extensive debridement of nonviable
epidermis, followed by immediate cover with biologic dressings, are among the
recommended treatments. Biologic dressings may include the following:
Porcine cutaneous xenografts
Cryopreserved cutaneous allografts
Amnion-based skin substitutes
Collagen-based skin substitutes
The ophthalmology literature supports concurrent coverage of the involved eye(s) with
amniotic membrane.[37]
Leaving the involved epidermis that has not yet peeled off in place and using biologic
dressings only on raw dermis also has been recommended. Skin allotransplantation
reduces pain, minimizes fluid loss, improves heat control, and prevents bacterial
infection. Hyperbaric oxygen can also improve healing.
Immunomodulatory Therapy
Stevens-Johnson syndrome is a rare disorder with relatively high mortality and morbidity
rates. To date, because of a lack of consensus on the proposed therapeutic modalities,
intensive supportive management and withdrawal of the offending drug remain the
criterion standard.
For any intervention, a prospective randomized controlled trial would be the most
appropriate step to validate its use. However, a large number of patients are required to
reach statistical significance. Furthermore, for ethical reasons, withdrawal of a potentially
life-saving therapy for the sake of randomization with a placebo control is not possible.
Several therapeutic modalities have been advocated for the treatment of Stevens-Johnson
syndrome based on the current, yet incomplete, understanding of its pathogenetic
mechanisms. Plasmapheresis, immunosuppressive therapy, and intravenous
immunoglobulin (IVIG) have been used with variably successful results.
The use of systemic steroids remains controversial. Some authors believe that they are
contraindicated, especially because there may be some question about the diagnosis.
Patients with infection-induced erythema multiforme do worse when steroids are given.
(Note that the differentiation between Stevens-Johnson syndrome and erythema
multiforme should be possible even in the acute stage.)[38] Prolonged treatment with
systemic steroids has been associated with an increased prevalence of complications.
However, concerns about the safety of systemic corticosteroids in the treatment of
Stevens-Johnson syndrome are based on a few case series; in those reports, systemic
corticosteroids were administered too late in the course of the disease, in inappropriately
low doses, and for a very long duration that actually impaired the healing process and
increased the risk of sepsis. The currently advocated approach for corticosteroid use
suggests the early use of short-term (4-7 days), high-dose intravenous corticosteroids.[39, 40]](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-14-320.jpg)
![The ophthalmology literature contains several papers that advocate systemic and topical
steroids to minimize ocular morbidity.[41, 42] Authors have cited salvage of vision when
pulse steroid therapy has been given.[38, 42] Others have concluded that IV steroids and
immunoglobulins do not improve outcome.[43]
The role of other immunosuppressive therapy, that is, cyclosporine, azathioprine, or
cyclophosphamide, in the acute phase is less popular, particularly since such medication
typically takes weeks to begin to influence immunological reactions. Cyclophosphamide
has been reported to be the culprit drug that induced Stevens-Johnson syndrome in one
instance.[44]
Nevertheless, the role of cyclosporine in the treatment of the acute phase of Stevens-
Johnson syndrome has been revisited, and, indeed, it showed encouraging results.[12] Also,
immunosuppressive therapy may play a pivotal role in the management of the chronic
ocular surface inflammation that can occur later on in selected cases.
The rationale for the use of IVIG is the most appealing. Based on in vitro and clinical
data, IVIG can block the Fas receptors on the surface of the keratinocytes, thus
interfering with the Fas-Fas ligand mediated apoptosis.[45] Encouraging results were
reported when IVIG was used in high doses very early in the course of the disease and for
a short period. Unfortunately, there is no consensus with regard to either the dose or the
duration of treatment with IVIG.[8]
Prophylactic use of IVIG has also been reported. One group used IVIG in a patient who
underwent cardiac catheterization but who had 4 previous Stevens-Johnson syndrome
episodes after intravenous contrast injection.[46]
However, a large European study designed to evaluate the efficacy of various treatments,
the EuroSCAR Study, "found no sufficient evidence of a benefit for any specific
treatment."[47] The group looked at mortality in patients treated with IVIG and
corticosteroids. However, in a letter to the editor, Pehr disagreed with the findings in the
EuroSCAR study citing inadequate doses of IVIG and corticosteroids in that study.[48]
Interestingly, few studies have addressed the effect of systemic steroids or IVIG on either
the development or the outcome of ocular manifestations in Stevens-Johnson syndrome
and toxic epidermal necrolysis (TEN). Neither treatment appeared to have an effect on
the ocular outcome in patients in two reports.[49, 4]
Treatment of Acute Ocular Manifestations
Treatment of acute ocular manifestations usually begins with aggressive lubrication of
the ocular surface. As inflammation and cicatricial changes ensue, most ophthalmologists
use topical steroids, antibiotics, and symblepharon lysis.
In case of exposure keratopathy, tarsorrhaphy may be required.
Maintenance of ocular integrity can be achieved through the use of amniotic membrane
grafting, adhesive glues, lamellar grafts, and penetrating keratoplasty, either in the acute
phase or in subsequent follow-up care.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-15-320.jpg)

![Patients with SJS require regular monitoring of their medications and status. Although
patients with erythema multiforme minor may be treated as outpatients with topical
steroids, those with erythema multiforme major (ie, Stevens-Johnson syndrome) must be
hospitalized. Cases of erythema multiforme minor must be followed closely. Some
authors recommend daily follow-up.
2- Toxic Epidermal Necrolysis
Toxic epidermal necrolysis (TEN) is a potentially life-threatening dermatologic disorder
characterized by widespread erythema, necrosis, and bullous detachment of the epidermis
and mucous membranes, resulting in exfoliation and possible sepsis and/or death (see the
image below). Mucous membrane involvement can result in gastrointestinal hemorrhage,
respiratory failure, ocular abnormalities, and genitourinary complications.
Diffuse maculopapular rash in toxic epidermal necrolysis (TEN).
TEN is most commonly drug induced. However, the disorder has other potential
etiologies, including infection, malignancy, and vaccinations (see Etiology). TEN is
idiosyncratic, and its occurrence is not easily predicted.
Some authors believe that Stevens-Johnson syndrome (SJS; also known as erythema
multiforme major) is a manifestation of the same process involved in TEN, with the latter
involving more extensive necrotic epidermal detachment. TEN involves more than 30%
of the body surface, whereas SJS involves less than 10% (see Differentials).
A classification system, based largely on the extent of epidermal detachment and
morphology of the skin lesions, aids in differentiating opposite spectrums of the same
disease entity.[1] This system comprises the following:
TEN with spots
TEN without spots
Overlap Stevens-Johnson syndrome and TEN (SJS-TEN)
TEN with spots is defined as widespread, irregularly shaped erythematous or purpuric
macules with blistering that occurs on all or part of the macule. Blisters become more
confluent and result in detachment of the epidermis and erosions on greater than 30% of
the body surface area. Mucosal surfaces are usually involved.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-17-320.jpg)
![TEN without spots is defined as widespread, large areas of erythema with no discrete
lesions. Epidermal detachment is greater than 10% of the body surface area. Mucosal
surfaces are usually involved.
Overlap Stevens-Johnson syndrome and TEN (SJS-TEN) is characterized by widespread,
irregularly shaped erythematous or purpuric macules with blistering that occurs on all or
part of the macule. Blisters become confluent and result in detachment of the epidermis
and erosions on 10-29% of the body surface area.
TEN is a clinical diagnosis, confirmed by histopathologic analysis of lesional skin (see
Clinical and Workup). The mainstay of treatment is supportive care until the epithelium
regenerates. Early transfer of patients to a burn or intensive care unit has been shown to
reduce the risk of infection, mortality rate, and length of hospitalization (see Treatment).
Historical background
Alan Lyell provided an early description of TEN in 1956, describing the condition as "an
eruption resembling scalding of the skin."[2] This dermatologic condition is characterized
by extensive epidermal loss suggestive of severe scalding. In that same year, Lang and
Walker reported a case of TEN.[3] The disorder was originally described by Debre et al in
1939 in French as l'erythrodermie bulleuses avec epidermolyse.[4]
Lyell later reclassified the conditions of 2 of his patients as having staphylococcal
scalded skin syndrome,[5] which is due to Staphylococcus aureus infection rather than to a
probable drug hypersensitivity-type reaction. Histopathologic analysis of the skin remains
the main tool for discrimination between the two conditions.
Patient education
Patients who have had TEN must be counseled regarding the likely causative medication
or agent, and they must be advised to avoid these medications and those of the same or
similar classes in the future. Cross-reactivity may occur with agents that chemically
resemble the causative agent. Patients must call a pharmacist whenever they start a new
prescription.
Genetic factors are suspected in drug-induced blistering disorders, and blood relatives of
the patient also should not use the suspected drug.
Pathophysiology
The pathophysiology of TEN has not been fully elucidated; however, various theories
have received wide acceptance. TEN is believed to be an immune-related cytotoxic
reaction aimed at destroying keratinocytes that express a foreign antigen.
TEN mimics a hypersensitivity reaction, with its characteristic delayed reaction to an
initial exposure and an increasingly rapid reaction with repeated exposure.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-18-320.jpg)
![The widespread epidermolysis and blistering of TEN results from keratinocyte
apoptosis—an organized series of biochemical reactions leading to cell changes and cell
death.[6] However, the number of inflammatory T cells in the skin of patients with TEN is
variable and perhaps too low to explain the widespread destruction.[7]
There is evidence supporting several immunopathologic pathways leading to keratinocyte
apoptosis in TEN, including the following:
Fas ligand activation on keratinocyte membranes leading to death receptor–
mediated apoptosis[8]
Release of destructive proteins (perforin and granzyme B) from cytotoxic T
lymphocytes (CTLs) generated from an interaction with cells expressing major
histocompatability complex (MHC) class I[9]
Overproduction of T cell– and/or macrophage-derived cytokines (interferon-γ
[INF-γ], tumor necrosis factor-α [TNF-α], and various interleukins)[10, 11]
Drug-induced secretion of granulysin from CTLs, natural killer cells, and natural
killer T cells[12]
Precisely how the inciting agent triggers the proposed pathways is yet to be elucidated.
Etiology
TEN can be induced by drugs or infection or can be idiopathic. Medications are the major
precipitating cause. Numerous medications have been implicated,[13] including antibiotics,
antiepileptic drugs, nonsteroidal anti-inflammatory drugs (NSAIDs), ampicillin,
allopurinol, corticosteroids (topical and systemic), and the antiretroviral drugs nevirapine
and abacavir.[14, 15]
Antibacterial drugs associated with TEN include the following:
Sulfonamides (4.5 cases per million users per week)
Chloramphenicol
Macrolides (eg, erythromycin)
Penicillins
Quinolones (eg, ciprofloxacin,[16] trovafloxacin[17] )
Anticonvulsants associated with TEN include the following:
Phenobarbital
Phenytoin[18]
Carbamazepine
Valproic acid
Lamotrigine
TEN in patients taking anticonvulsants has most often been reported within 2 months of
starting the drug. However, some cases associated with long-term use have been reported.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-19-320.jpg)
![NSAIDs associated with TEN include the following:
Phenylbutazone and oxybutazone - Implicated most commonly, although they are
no longer available in the United States
Oxicams (eg, piroxicam, tenoxicam) - Implicated more often than other NSAIDs
Ibuprofen
Indomethacin
Sulindac
Tolmetin
With allopurinol, risk is not constant over time. Patients have a 5.5 relative risk.
However, during the first 2 months of therapy, the relative risk is 52, and the long-term
therapy risk is 0.5.
No laboratory test is able to confirm a specific drug etiology. A causal link is suggested
when TEN occurs during the first 4 weeks of medication therapy, usually between 1 and
3 weeks. Drugs with longer half-lives and those with circulating active metabolites may
result in more fulminant disease.
Infectious agents (ie, Mycoplasma pneumoniae, herpes virus, hepatitis A),
immunizations, and bone marrow or solid organ transplantation have also been associated
with TEN.
Epidemiology
In the United States, the annual frequency of TEN is reported to be 0.22-1.23 cases per
100,000 population. In the HIV-positive population, the incidence of TEN increases to 1
case per thousand per year.[19]
Worldwide, the average annual incidence of TEN is 0.4-1.3 cases per million
population.[20] In 1992, the cumulative incidence of TEN and SJS in Germany was 1.9
cases per million population. A French survey of dermatologists and health care facilities
reported an annual incidence of 1 case per million population.
Race-, sex-, and age-related demographics
A genetic predilection toward carbamazepine-induced TEN has been observed in HLA-B*
1502–positive Han Chinese patients.[21] The US Food and Drug Administration
recommends screening for the HLA-B*1502 allele before initiating carbamazepine in
patients of Asian ancestry.[22]
For unclear reasons, TEN appears to have a predilection for females. The female-to-male
ratio is 1.5:1.[23]
TEN may occur in all age groups; however, the mean age of patients with TEN is
reported to be between 46 and 63 years. Infection is more commonly implicated as an
etiology in children, whereas medication exposure is more common in adults. Elderly
persons may be at greater risk because of their tendency to use multiple medications.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-20-320.jpg)
![Prognosis
The estimated mortality associated with TEN varies widely in different reports, from 10-
70%. Outcome depends in part on the quality of care and the rapidity with which
treatment is initiated.
Septicemia and multisystem organ failure are the primary causes of death. Epithelial loss
results in vulnerability to bacterial and fungal infections. Sloughing of stratified
epithelium of mucosal membranes can result in GI hemorrhage, respiratory failure, ocular
abnormalities, and genitourinary lesions. Significant fluid loss from extensive skin
exfoliation and an inability to tolerate oral intake can lead to hypovolemia, acute tubular
necrosis, and shock.
Age, extent of epidermal involvement, and serum urea level are said to be the most
important prognostic factors in TEN.[24] Mortality rates in children are much lower than in
adults.[25] Elderly patients have a poor prognosis.
Other negative prognostic factors include the following:
Elevated blood urea nitrogen (BUN) and serum creatinine levels
Respiratory failure
Multiple drugs
Thrombocytopenia
Lymphopenia
Neutropenia
Leukopenia
Sepsis
Severity-of-illness score
A severity-of-illness score that estimates the risk of death in TEN (SCORTEN) has been
developed and validated.[26] Each of the following independent prognostic factors is given
a score of 1:
Age >40 years
Heart rate >120 beats per minute
Cancer or hematologic malignancy
Involved body surface area >10%
Blood urea nitrogen level >10 mmol/L (28 mg/dL)
Serum bicarbonate level < 20 mmol/L (20 mEq/L)
Blood glucose level >14 mmol/L (252 mg/dL)
The number of positive criteria and the corresponding mortality rates are as follows:
0: 1 to 3%
2: 12%
3: 35%](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-21-320.jpg)
![ 4: 58%
5 or more: 90%
Treatment
Prehospital care for patients with TEN is similar to that for patients with burns.
Supplement with oxygen by face-mask as needed; do not perform prophylactic tracheal
intubation. Prevent hypothermia with rewarming devices and blankets.
In severe TEN, the barrier function of the skin is compromised. Thus, contamination and
evaporation must be minimized. The patient should be treated similarly to one with
extensive burns, that is, with the application of sterile coverings. Fluid and pulmonary
status must be carefully monitored.
Emergency Department Care
For the emergency physician, the two most important elements in the treatment of TEN
are discontinuation of the offending drug and admission to a burn unit.[33] Evidence
suggests that rapid institution of these two measures is associated with a more favorable
prognosis.[34, 35]
Emergency department care should be directed toward the following:
Maintaining fluid and electrolyte homeostasis
Mitigating temperature loss
Providing adequate analgesia
Preventing secondary infection
Aggressive fluid and electrolyte management, pain control, and meticulous skin care are
important. Fluid resuscitation with crystalloids should follow standard guidelines used for
burn patients. However, patients with TEN typically require less aggressive fluid
replacement than that of burn patients because of less severe microvascular injury.
A goal of resuscitation should be to maintain sufficient mean arterial blood pressure
(ABP >65 mm Hg), central venous pressure (CVP 8-12 mm Hg), and central oxygenation
(Svco2 >70%) for adequate tissue perfusion and renal perfusion.[23] Fluid management
should be based on the physiologic endpoint of urine output of 0.5-1 mL/kg/h.[33]
Patients with extensive skin involvement require reverse isolation and a sterile
environment. Areas of skin erosion should be covered with nonadherent protective
dressings such as petrolatum gauze. Respiratory distress may result from mucosal
sloughing and edema and may necessitate endotracheal intubation and ventilation.
Approach Considerations](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-22-320.jpg)
![Management of toxic epidermal necrolysis (TEN) requires prompt recognition of the
disorder and withdrawal of all potential causative agents. The mainstay of treatment is
supportive care until the epithelium regenerates. Supportive measures include isolation,
fluid and electrolyte balance, nutritional support, pain management, and protective
dressings. Early transfer of patients to a burn or intensive care unit has been shown to
reduce the risk of infection, mortality rate, and length of hospitalization.
Withdraw the offending agent, if one is identified, as soon as possible. One observational
study showed a reduction in mortality from 26% to 5% when the implicated drugs with
short elimination half-lives were withdrawn no later than the day the blisters or erosions
first developed.
No controlled prospective treatment studies or generally accepted guidelines exist. In
1991, Avakian and colleagues published the University of Florida treatment protocol for
toxic epidermal necrolysis.[31] In 2007, these guidelines were revised by Fromowitz and
colleagues.[32] The guidelines are as follows:
Monitor fluids and electrolytes. Administer fluids and titrate based on central venous
pressure and urine output; on average, 3-4 L are needed in patients with 50% of the body
surface area affected.
Parenteral nutrition or nutrition provided enterally via a soft-fine bore nasogastric tube is
usually needed. Start total parenteral nutrition in patients unable to take nourishment.
Early and continuous enteral nutrition reduces the risk of stress ulcers, reduces bacterial
translocation and enterogenic infection, and allows earlier discontinuation of venous
lines.
Supportive Systemic Therapy
The patient should be placed in a heated environment to enhance reepithelialization.
However, this may enhance water losses, and appropriate hydration must be maintained.
Institute a bed warmer.
Saline applied to skin hourly is important, and then emollients are smeared.
Chlorhexidine solution is used to bathe the patient's skin. Chlorhexidine mouthwash is
administered 4 times a day, and white petrolatum is administered to the lips. Rinse the
patient’s mouth frequently and apply a topical anesthetic or spray for buccal pain.
Provide daily physical therapy for range-of-motion exercises.
Place a Foley catheter and nasogastric tube only when needed.
Energy requirements for patients with TEN must be carefully calculated and nutritional
support provided. Protein loss can be significant.[25]
Patients with mucosal vulnerability may have severe bleeding complications. Coagulation
factors and blood counts should be held within the normal ranges, and transfusion of red
cells, platelets, and plasma products should be considered when necessary.[23]](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-23-320.jpg)

![ Corticosteroids
Cyclophosphamide
Cyclosporine
Tumor necrosis factor–alpha (TNF-alpha) inhibitors
Intravenous immune globulin (IVIg)[6, 36]
Multiple studies of these modalities have been completed, and multiple studies are
ongoing. Completed studies have shown that either the risk of the medication outweighs
the benefit or the data are inconclusive to support its utilization. Therefore, there is a
significant need for randomized control studies to further evaluate potential treatment
modalities in TEN.
Corticosteroids are commonly used to control progression of TEN, but this is highly
controversial. In some studies, corticosteroids have increased the incidence of mortality.
Consider the use of plasmapheresis, if available, daily for 3 days. Although prospective
randomized studies have not been performed, limited data suggest that plasmapheresis
may enhance elimination of the drug or offending agent or inflammatory mediators such
as cytokines and should be considered.[37]
Anti–TNF-α treatment has been reported to rapidly resolve skin lesions due to TEN.
TNF-α is strongly expressed in keratinocytes and macrophages of lesional skin, and high
concentrations are found in cutaneous blister fluid.
Sucrose-depleted IVIg 1 g/kg/d (infused over 4 h) for 3 days may be beneficial if started
within 48-72 hours of bulla onset. If more than 72 hours have elapsed since the onset of
bulla but TEN is still actively progressing, with new lesions, IVIg may still be useful.
Consultations
Most patients with TEN require specialized care under the direction of a team of
physicians with experience in handling this disorder. Burn-unit care represents an option
worthy of serious consideration.
Required consultations may include the following:
A dermatologist is consulted to identify and to confirm the diagnosis of TEN
A plastic surgeon is consulted to debride areas of skin necrosis, as indicated
An ophthalmologist is consulted for assisting in the treatment of ocular
manifestations and preventing long-term sequelae
An internal medicine specialist is consulted to assist in patient treatment
Consultation with a respiratory medicine specialist may be important, since
respiratory mucosa may slough; establishment of pulmonary toilet may be
advisable
Otolaryngologic or urologic consultation may be helpful in patients with
significant mucous membrane involvement of those areas.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-25-320.jpg)
![Sequelae
Major sequelae are generally limited to the affected organ systems (ie, the skin and
mucosal membranes).
Cutaneous sequelae of TEN include the following:
Changes in skin pigment (hypopigmentation or hyperpigmentation; sun exposure
must be avoided for several months because ultraviolet light can worsen
hyperpigmentation; sunblock is recommended)
Nail loss and nail dystrophy
Hypohidrosis (inability to sweat)
Scarring, alopecia, and hypertrophic scarring
Dermal desiccation, causing deep dermal wounds
Chronic xerostomia
Esophageal strictures
Vulvovaginal synechiae
Phimosis
Chronic erosion of the mouth and genitalia
Ocular complications generally result from abnormal keratinization of the tarsal
conjunctiva. A Sjogrenlike syndrome with decreased lacrimal secretion causes dry eye
and predisposes to corneal abrasions and corneal scarring with neovascularization. In
addition, patients have been reported to have palpebral synechiae, entropion, or
symblepharon (adhesion of the eyelids).[27]
A study by Power and colleagues found that 50% of patients with TEN developed ocular
complications.[28] Patients treated with steroids fared no better than those treated without
steroids. Therefore, TEN remains a common cause of visual loss in a significant number
of patients. Ultimately, 5-9% of patients can become blind as a result of some of these
complications.
3-Erythema Multiforme
Erythema multiforme (EM) is an acute, self-limited, and sometimes recurring skin
condition that is considered to be a type IV hypersensitivity reaction associated with
certain infections, medications, and other various triggers.[1]
Erythema multiforme may be present within a wide spectrum of severity. Erythema
multiforme minor represents a localized eruption of the skin with minimal or no mucosal
involvement. The papules evolve into pathognomonic target lesions or iris lesions that](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-26-320.jpg)
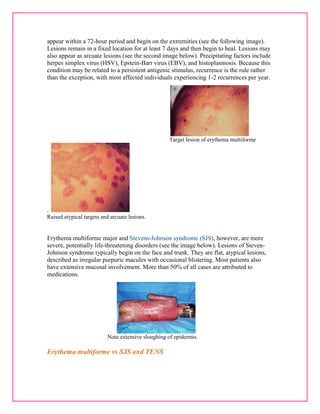
![Controversy exists in the literature with regard to the clinical definitions of erythema
multiforme and Steven-Johnson syndrome and whether they are distinct entities or
whether they represent a spectrum of one disease process.[2, 3, 4, 5, 6, 7] International
collaborators have suggested that erythema multiforme and Steven-Johnson syndrome
could be separated as 2 distinct clinical disorders with similar mucosal reactions but
different patterns of cutaneous lesions.
The confusion between these 2 separate clinical entities began in 1950, when Thomas
coined the terms erythema multiforme minor and erythema multiforme major to describe
conditions he encountered. Erythema multiforme minor was applied to patients with the
illness originally described by Ferdinand von Hebra as erythema multiforme (acute, self-limited
condition with characteristic red papular skin lesions) (1860).[2] Erythema
multiforme major was applied to patients who also displayed oral mucosal involvement,
similar to that described by Stevens and Johnson (mucocutaneous disorder; febrile
erosive stomatitis, severe conjunctivitis, and disseminated cutaneous eruption) (1922).[3]
Up to 50% of patients with herpes simple virus (HSV)–associated erythema multiforme
have been found to have oral ulcers. However, this is now recognized as a variant of
erythema multiforme, rather than Steven-Johnson syndrome. Erythema multiforme and
Steven-Johnson syndrome have different precipitating factors and different clinical
patterns and are generally recognized to be separate clinical entities. Erythema
multiforme with mucosal involvement is now termed bullous erythema multiforme.
Consensus classification
According to a consensus definition, Steven-Johnson syndrome was separated from the
erythema multiforme spectrum and added to toxic epidermal necrolysis.[3] Essentially
Steven-Johnson syndrome and toxic epidermal necrolysis (TEN) are considered severity
variants of a single entity. The 2 spectra are now divided into the following: (1) erythema
multiforme consisting of erythema minor and major and (2) Steven-Johnson syndrome /
toxic epidermal necrolysis (SJS/TEN).
The clinical descriptions are as follows:
Erythema multiforme minor - Typical targets or raised, edematous papules
distributed acrally
Erythema multiforme major - Typical targets or raised, edematous papules
distributed acrally with involvement of one or more mucous membranes;
epidermal detachment involves less than 10% of total body surface area (TBSA).
SJS/TEN - Widespread blisters predominant on the trunk and face, presenting
with erythematous or pruritic macules and one or more mucous membrane
erosions; epidermal detachment is less than 10% TBSA for Steven-Johnson
syndrome / toxic epidermal necrolysis and 30% or more for toxic epidermal
necrolysis.
Historical information](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-28-320.jpg)
![Stevens-Johnson syndrome was considered an extreme variant of erythema multiforme
for many years, whereas toxic epidermal necrolysis (TEN) was considered a different
entity. However, in 1993, a group of medical experts proposed a consensus definition and
classification of erythema multiforme, Steven-Johnson syndrome, and toxic epidermal
necrolysis based on a photographic atlas and extent of body surface area involvement.[3]
Pathophysiology
The pathophysiology of erythema multiforme (EM) is still not completely understood,
but it is probably immunologically mediated and appears to involve a hypersensitivity
reaction that can be triggered by a variety of stimuli, particularly bacterial, viral, or
chemical products.
Cell-mediated immunity appears to be responsible for the destruction of epithelial cells.
Early in the disease process, the epidermis becomes infiltrated with CD8 T lymphocytes
and macrophages, whereas the dermis displays a slight influx of CD4 lymphocytes. These
immunologically active cells are not present in sufficient numbers to be directly
responsible for epithelial cell death. Instead, they release diffusable cytokines, which
mediate the inflammatory reaction and resultant apoptosis of epithelial cells. In some
patients, circulating T cells transiently demonstrate (for < 30 d) a T-helper cell type 1
(TH1) cytokine response (interferon [IFN] gamma, tumor necrosis factor [TNF] alpha,
interleukin [IL] 2). Results of immunohistochemical analysis have also shown lesion
blister fluid to contain TNF, an important proinflammatory cytokine.
Other evidence supports the hypothesis that the disease is the result of cell-mediated
immune reactions. Individuals possessing human leukocyte antigen (HLA)–B12 are 3
times more likely to develop this disorder. The classic timing for a primary cell-mediated
immune reaction is 9-14 days after the initiation of the offending drug. In recurrent
exposure, the reaction occurs within several hours to 1-2 days, which is consistent with
the timing of a secondary cell-mediated immune response.
Herpes simplex virus
A major cause of erythema multiforme is the herpes virus (HSV). In fact, recent or
recurrent herpes has been reported as the principle risk factor for erythema multiforme.
Herpes-associated erythema multiforme (HAEM) appears to represent the result of a cell-mediated
immune reaction associated with HSV antigen.[8, 9] The immunologic reaction
affects HSV-expressing keratinocytes. Cytotoxic effector cells, CD8+ T lymphocytes in
the epidermis, induce apoptosis of scattered keratinocytes and lead to satellite cell
necrosis. Neighboring epidermal cells are HLA-DR positive.
A relationship exists between HLA types A33, B35, B62 (B15), DR4, DQB1*0301,
DQ3, and DR53 and recurrent erythema multiforme.[10] In particular, HLA-DQ3 is
especially related to recurrent erythema multiforme and may be a helpful marker for
distinguishing HAEM from other cutaneous diseases.[11]](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-29-320.jpg)
![Drug hypersensitivity
The disease process also often involves an abnormal metabolism of a responsible drug.
As noted above, the keratinocyte is the ultimate target of this disease process, with
keratinocyte necrosis being the earliest pathologic finding.
Patients frequently display an altered metabolism of the responsible drug, and are
considered to be slow acetylators, both genotypically and phenotypically. This means that
an increased proportion of drug metabolism is directed toward the alternative pathway of
oxidation by the cytochrome P-450 system, resulting in increased production of reactive
and potentially toxic metabolites. Affected individuals have a defect in the ability to
detoxify these reactive metabolites, which may then behave as haptens by binding
covalently to proteins on the surface of epithelial cells. This may then induce the immune
response, leading to the severe skin reaction.
Etiology
Many suspected etiologic factors have been reported to cause erythema multiforme (EM).
Both erythema multiforme and Steven-Johnson syndrome may be induced by
medications, but infectious agents are also considered to be a major cause of erythema
multiforme. However, approximately 50% of cases are idiopathic, with no precipitating
factor identified.
A previous history of erythema multiforme and male sex has also been reported as risk
factors, but pregnancy may contribute to development of erythema multiforme as well.
Postvaccination causes include Bacille Calmette-Guérin (BCG) vaccination, oral polio
vaccine, vaccinia, and tetanus/diphtheria.
HSV and other infections
Infectious causes are more common in children and are implicated more commonly in
erythema multiforme.
Erythema multiforme minor is regarded as being commonly triggered by herpes simplex
virus (HSV) (types 1 and 2), and HSV is the most common cause in young adults; in fact,
many instances of idiopathic erythema multiforme minor may be precipitated by
subclinical HSV infection. Among other infections, Mycoplasma species appear to be a
common cause.
Bacterial
Bacterial infections include borreliosis, catscratch disease, diphtheria, hemolytic
streptococci, legionellosis, leprosy, Neisseria meningitidis, Mycobacterium avium
complex, M pneumoniae,[12, 13] pneumococci, tuberculosis,
Proteus/Pseudomonas/Salmonella/Staphylococcus/Yersinia species, Treponema
pallidum,[14] tularemia, Vibrio parahaemolyticus, Vincent disease, and rickettsial
infections. Chlamydial infections include lymphogranuloma venereum and psittacosis.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-30-320.jpg)
![Viral
Viral infections include Adenovirus, coxsackievirus B5, cytomegalovirus (CMV),
echoviruses, enterovirus, Epstein-Barr virus (EBV), hepatitis A / B / C viruses (HAV /
HBV / HCV), HSV, influenza,[15] measles, mumps, paravaccinia, parvovirus B19,
poliomyelitis, varicella-zoster virus (VZV), and variola.
Virus-drug interactions include CMV infection–terbinafine[16] and EBV infection–
amoxicillin.[17]
Other
Fungal infections include coccidioidomycosis, dermatophytosis, and histoplasmosis.
Parasitic infections include Trichomonas species and Toxoplasma gondii.
Drugs
More than 50% of cases are related to medication use, but no test reliably proves the link
between a single case and a specific drug.
Regarding medications, sulfa drugs are the most common triggers (30%). A slow
acetylator genotype is a risk factor for sulfonamide-induced Steven-Johnson syndrome.[18]
The second most commonly involved agents are the anticonvulsants, including
barbiturates,[19] carbamazepine,[19] hydantoin, phenytoin,[19] and valproic acid. Prophylactic
anticonvulsants after surgery for a brain tumor combined with cranial irradiation may
result in life-threatening Steven-Johnson syndrome.[20]
Causative antibiotics include penicillin, ampicillin, tetracyclines, amoxicillin, cefotaxime,
cefaclor, cephalexin, ciprofloxacin,[21] erythromycin, minocycline, sulfonamides,
trimethoprim-sulfamethoxazole, and vancomycin.
Antituberculoid agents such as rifampicin, isoniazid, thiacetazone, and pyrazinamide are
also known offenders. Antipyretic agents as triggers include analgesics, especially aspirin
as well as phenylbutazone, oxyphenbutazone, and phenazone.
Others drugs that may cause erythema multiforme include acarbose, albendazole,
allopurinol,[19] arsenic, bromofluorene, quinine (Chinine), cimetidine, clofibrate,
corticosteroids, diclofenac, didanosine, dideoxycytidine, diphosphonate, estrogen,
etretinate, fluconazole, griseofulvin,[22] gabapentin, granulocyte-macrophage colony-stimulating
factor (GM-CSF), hydralazine, indapamide, indinavir, lamotrigine,[19]
methazolamide, mefloquine, methotrexate, meprobamate, mercurials, minoxidil,
nifedipine, nevirapine,[19] nitrogen mustard, nystatin, nonsteroidal anti-inflammatory drugs
(NSAIDs), phenolphthalein, piroxicam,[19] pyritinol, progesterone, potassium iodide,
sulindac, suramin, saquinavir, thiabendazole, thiouracil, terbinafine, theophylline,
verapamil, and dihydrocodeine phosphate.[23]
Contact exposure](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-31-320.jpg)
![Contactants include ammoniated mercury, budesonide, bufexamac, capsicum,
chloromethylnaphthalene, desoximetasone, dinitrochlorobenzene (DNCB), disperse blue
124, diphenylcyclopropenone, fire sponge (Tedania ignis), herbal medicines (eg, Alpinia
galanga),[24] isopropyl-p -phenylenediamine of rubber, nickel, nitrogen mustard,
oxybenzone, phenylbutazone, poison ivy,[25] proflavin, resin, rosewood, and triamcinolone
acetonide.
Other etiologic factors
The following have also been reported as causes of erythema multiforme:
Flavorings and preservatives, such as benzoic acid and cinnamon[26]
Immunologic disorders, such as transient selective C4 deficiency of infancy,[27]
collagen diseases, vasculitides, sarcoidosis, non-Hodgkin lymphoma, leukemia,
multiple myeloma, myeloid metaplasia, and polycythemia
Physical or mechanical factors, such as tattooing, radiotherapy, cold, and sunlight
Foods, including salmon berries and margarine
Malignancy
Hormonal
Epidemiology
The exact incidence of erythema multiforme (EM) in the United States is unknown;
however, as many as 1% of dermatologic outpatient visits are for erythema multiforme.
Globally, the frequency of erythema multiforme is estimated at approximately 1.2-6 cases
per million individuals per year.
Before the human immunodeficiency virus (HIV) epidemic among young males, there
was a slight female predominance of this disease. However, erythema multiforme is
currently more common in younger males (male-to-female ratio, range of 3:2 to 2:1)
(mainly second to fourth decades, but can include children and adolescents [20%][28] ).
The condition is rare in children younger than 3 years and in adults older than 50 years.
The following medical conditions seem to predispose individuals to a higher risk of
developing the disorder: HIV infection, corticosteroid exposure, bone marrow transplant,
systemic lupus erythematosus (SLE), graft versus host disease (GVHD), and
inflammatory bowel disease (IBD). Individuals undergoing radiation, chemotherapy, or
neurosurgery for brain tumors are also at higher risk.
Prognosis
Most cases of erythema multiforme (EM) are self-limited. In erythema multiforme minor,
the lesions evolve over 1-2 weeks and ultimately subside within 2-3 weeks without
scarring. However, the recurrence of erythema multiforme minor is common (up to one
third of cases) and mostly preceded by apparent or subclinical herpes simplex virus
(HSV) infection.](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-32-320.jpg)




![ Dermatologist: For diagnosis and management and for performance of skin
biopsies, if indicated
Internal medicine specialist or a pediatric specialist: For evaluation of the
underlying causes of disorders and systemic sequelae
Ophthalmologist: For early consultation in the evaluation and management of
ocular involvement; daily examinations for signs of ocular involvement; if
necessary, disruption of synechiae can be accomplished by administration of
wetting or antibiotic eyedrops
Burn or trauma surgeon: For familiarity with caring for critically ill patients with
burn wounds who have open wounds
Infectious disease specialists: For evaluation of intercurrent infections and
treatment recommendations
Respiratory therapist: For tracheobronchial involvement
Physical or occupational therapists
Psychologists, psychiatrists, or social workers may be helpful.
Monitoring and Prevention
The medical professional(s) who treated the patient during hospitalization should see the
patient regularly and provide symptomatic relief, as needed. Such practitioners may
include burn or trauma surgeons, ophthalmologists, nephrologists, infectious disease
specialists, and gastroenterologists.
The affected skin should be protected from any pressure or shear forces. Otherwise, early
institution of physical and occupational therapies is appropriate. To reduce the likelihood
of developing hyperpigmentation, recommend the use of sunscreens for 1 year after the
incident has resolved.
Topical corticosteroids are useful for outpatient treatment of patients with limited disease.
Prophylaxis for recurrence of herpes-associated erythema multiforme (HAEM) should be
considered in patients with more than 5 attacks per year; oral acyclovir may be helpful in
reducing recurrence (see Medications). Prophylaxis may be required for 6-12 months or
longer. If the patient's condition is unresponsive, continuous therapy with valacyclovir
has been reported to be effective.[30]
Tamoxifen may prevent premenstrual erythema multiforme.
Prophylactic antibiotics are not recommended because of the increased likelihood of
selecting out resistant strains. However, evidence of infection should lead to prompt
culturing and the selection of appropriate antimicrobial therapy based on culture and
sensitivity results. Some authors recommend routine alternate-day skin biopsy for culture
to distinguish simple skin colonization from true infectious invasion and to guide
antimicrobial therapy.
Once erythema multiforme (EM) due to a drug has been diagnosed, the patient should
never be rechallenged with the same drug or any other drug of the same class or similar
chemical structure. Chemically related compounds often share a common metabolic](https://image.slidesharecdn.com/oralmed-140825102720-phpapp01/85/Muco-cutaneo-ocular-syndrome-37-320.jpg)
