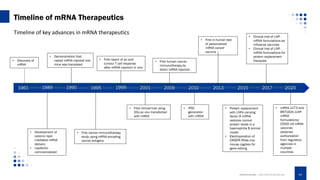
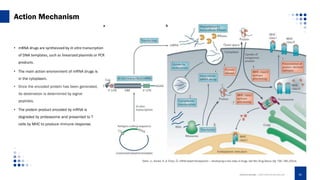
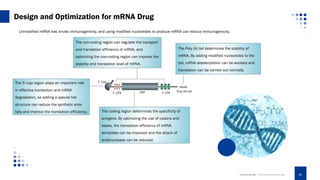
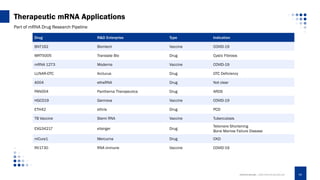
This document provides an overview of mRNA-based therapeutics, detailing the definition, action mechanism, and key advances in the field since 1961. It highlights the structure of mRNA, its therapeutic applications in various diseases, and the strategies for effective mRNA delivery and optimization. Additionally, Creative Biolabs offers services related to mRNA synthesis and development for various therapeutic applications.