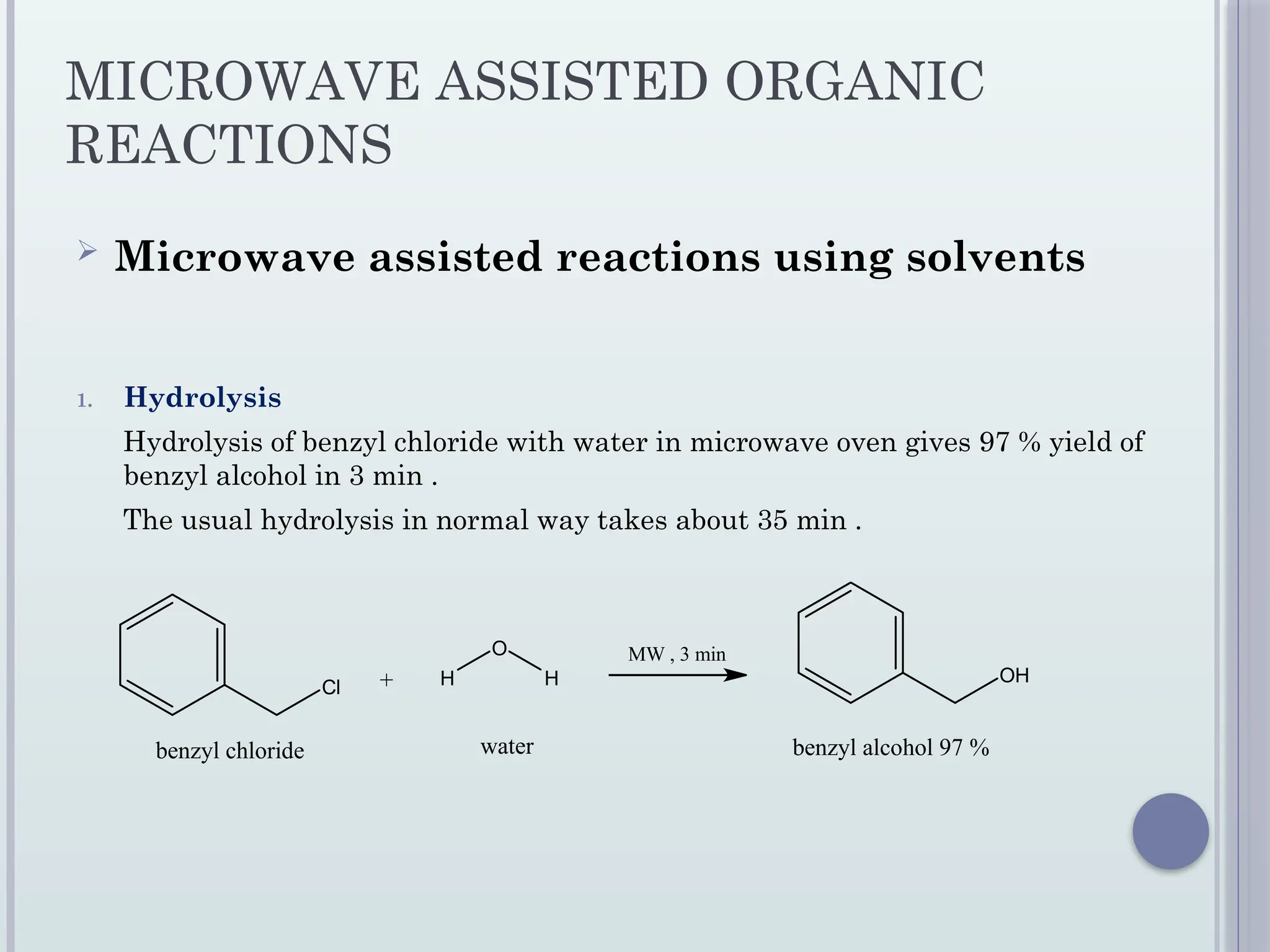
The document presents a seminar on microwave-assisted synthesis as a green chemistry approach, outlining the technique's principles, merits, and applications. It highlights faster reaction times, energy savings, and higher yields while acknowledging potential hazards and costs. The conclusion emphasizes the technique's efficiency and adaptability in various fields, including medicinal chemistry and material sciences.







![ Microwave assisted reactions using solid liquid
phase
1. O-Alkylation
Preparations of ethers were carried out from β-napthol using benzyl bromide and
1-butyl-3-methyl-imidazolium tetrafluoroborate under micrpwave irradiation
(6-12 min) the products were isolated in 75-90 % yields .
2. Oxidation
Oxidation of secondary alcohols to acetone derivatives was carried out using
tetrabutylammonium bromide and dichloromethane under microwave irradiation
(6-8 min) , products were isolated in 70-99 5 yields .
R1
OH
R2
PCC, TBAB , MWI
DCM , 6-8 min , 70-99 %
R1
O
R2
OH
BzBr , [BMm] BF
6-12 min , 75-90 %
O](https://image.slidesharecdn.com/microwaveassistedsynthesis-241223123113-44d04afd/75/Microwave-assisted-Synthesis-Garima-singh-pptx-9-2048.jpg)




