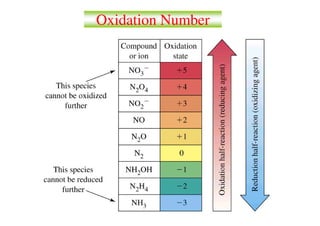
Here are the oxidation numbers of the requested elements in the given compounds:
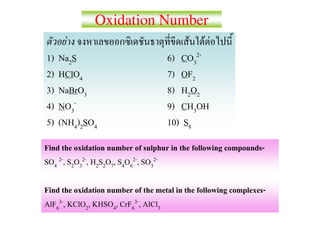
1) Na2S - S has an oxidation number of -2
2) HClO4 - Cl has an oxidation number of +7
3) NaBrO3 - Br has an oxidation number of +5
4) NO3- - N has an oxidation number of +5
5) (NH4)2SO4 - S has an oxidation number of +6
6) CO32- - C has an oxidation number of +4
7) OF2 - O has an oxidation number of -1
8) H2O2 - O has an oxidation number of -1
9) CH3OH -






![Oxidation Number
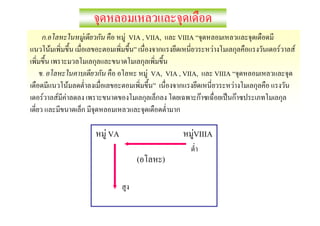
F 1 S H2SO4
F 2 Co [Co(CN)6]4 -
S=x
Co = x
H = +1
CN- = -1
2 H = (+1 × 2) = +2
CN = (-1 × 6) = -6
O = -2
F
4 O = (-2 ×4) = -8
F -4
F 0
x + (-6) = -4
+2 + x + (-8) = 0
x = +2
x = +6
Co [Co(CN)6]4- = +2
S H2SO4 = +6](https://image.slidesharecdn.com/microsoftpowerpoint-100518121048-phpapp01/85/Microsoft-power-point-25-320.jpg)