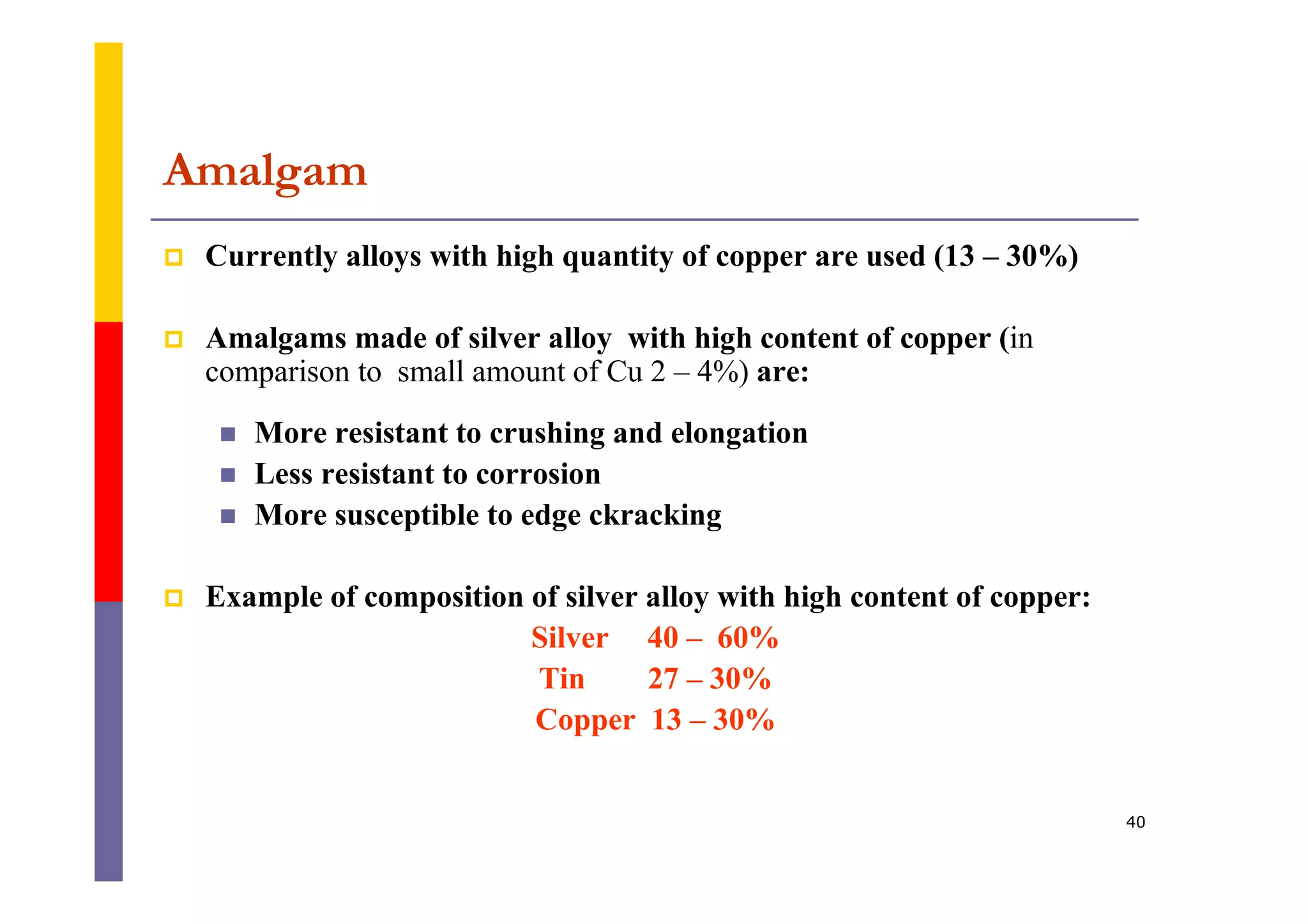
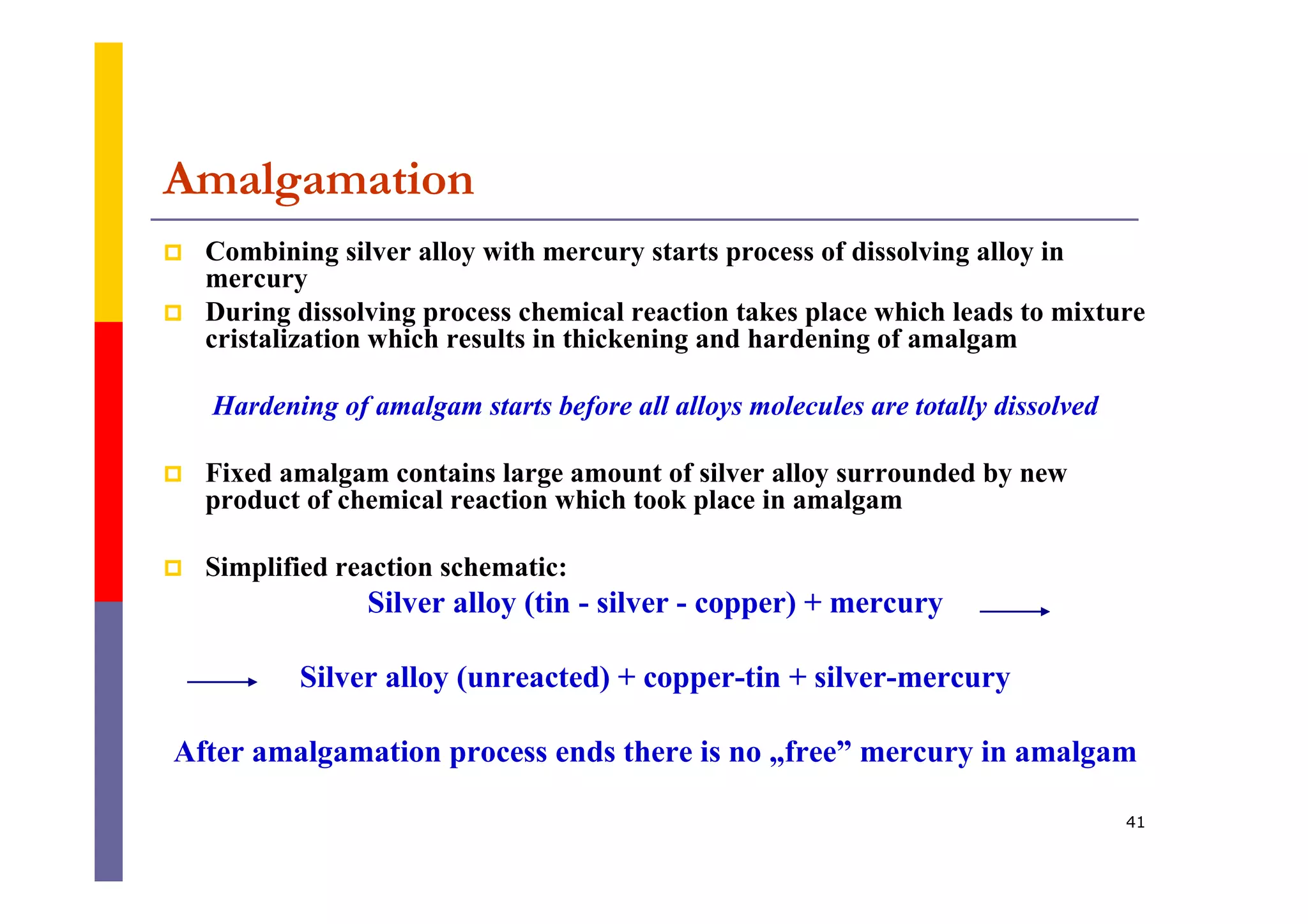
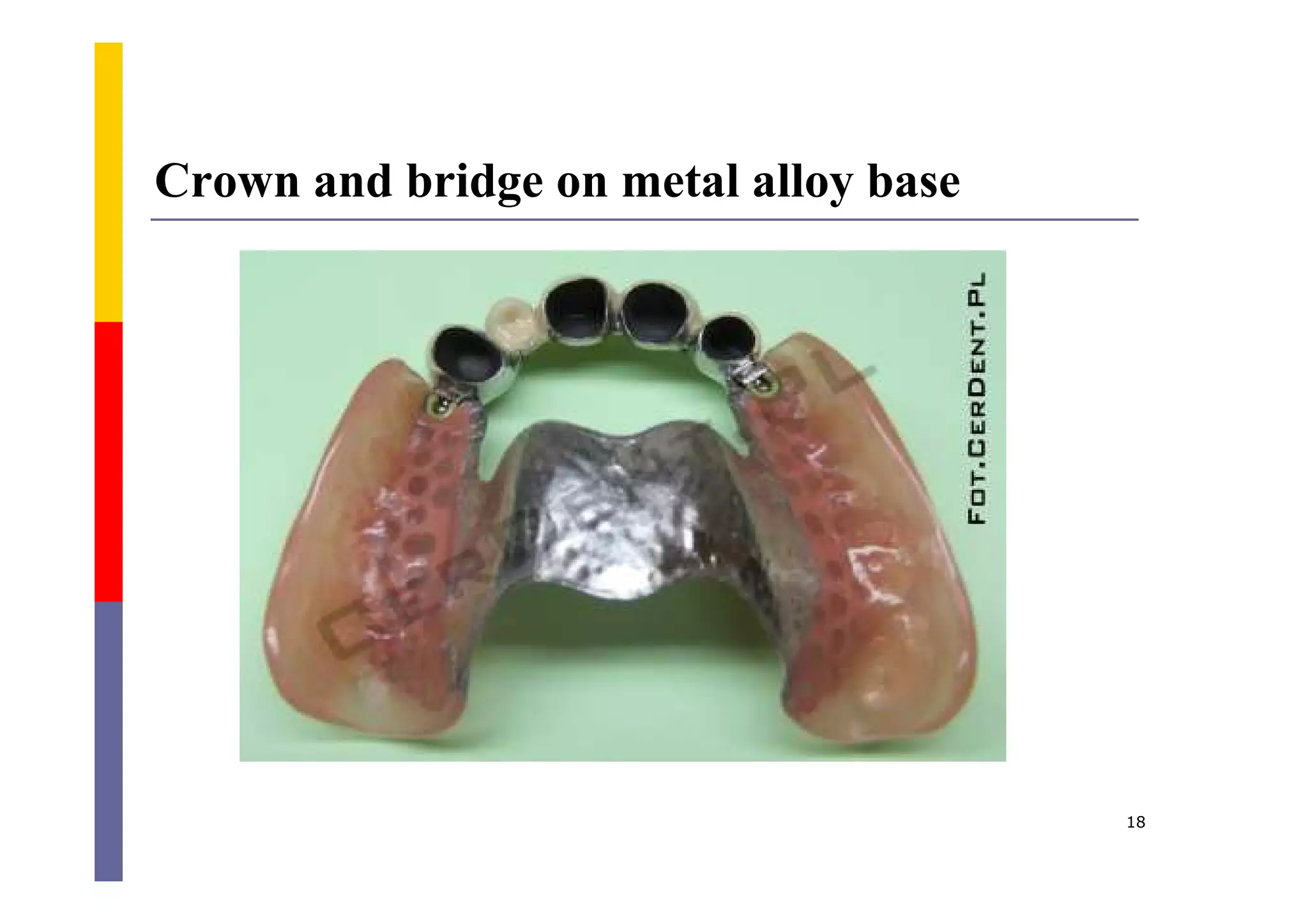
Metals are commonly used in dentistry due to their hardness, strength, and corrosion resistance. The main metals used are gold, platinum, palladium, silver, and titanium. Gold is often used on its own or in alloys due to its biocompatibility, corrosion resistance, and ability to form solid solutions with other metals. This changes the properties of gold for different applications. Titanium is also widely used for implants and prosthetics due to its strength, corrosion resistance, and ability to integrate with bone. Dental amalgam is a mixture of silver alloy powder and liquid mercury that hardens into a filling material.






























![32
Metal alloys – classification criteria according to ANSI/ADA
(American National Standards Institute /American Dental Association)
< 25any
With advantage of
noble metals
> 25anyNoble
> 60> 40Very noble
Nobel metal
content
Gold content
[weight %]
Alloy type](https://image.slidesharecdn.com/metalsindentistry-171217101945/75/Metals-in-dentistry-32-2048.jpg)



![36
Metal alloys – alloy density
Density of dental alloys are in the range from 4,5 g/cm3 (titanium alloys)
to 18,5 g/cm3 (some nobel alloys)
7,5
7,5
4,5
1275
1400 – 1500
1700
With excess of nobel
metals
Based on nickel
Based on cobalt
Based on titanium
12,4
10,6
10,6
865 – 925
1100 – 1190
1020 – 1100
Nobel
Silver- gold- copper
Palladium – copper
Silver -palladium
18,5
15,6
1045 – 1140
910 – 1065
Higly nobel
Gold - platinum
Gold- copper- silver
Density [g/cm3]Melting range [oC]Alloy type](https://image.slidesharecdn.com/metalsindentistry-171217101945/75/Metals-in-dentistry-36-2048.jpg)

![38
Metal alloys – hardness
Influences polish of alloy
Hardness is related to yield point.
Hardness is express in kg/mm2.
-it means amount of mass [kg] needed to be apply to make
notch of 1 mm2 area
hardness of dental alloys is in the range:125 – 425 kg/mm2
Enamel hardness is 343 kg/mm2](https://image.slidesharecdn.com/metalsindentistry-171217101945/75/Metals-in-dentistry-38-2048.jpg)