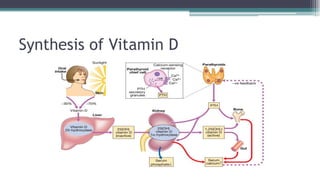
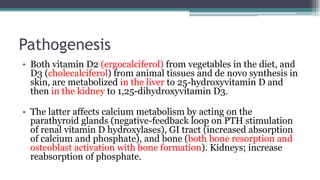
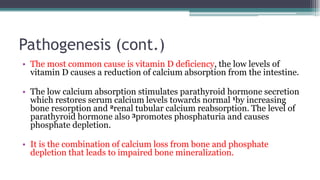
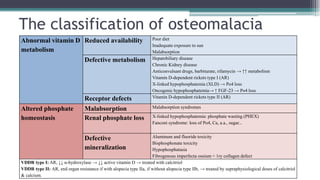
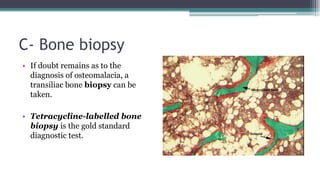
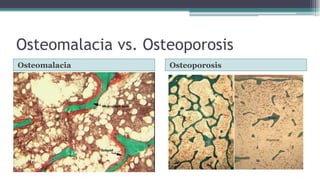
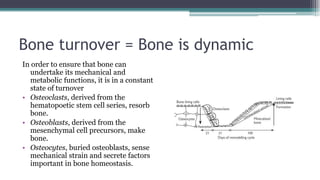
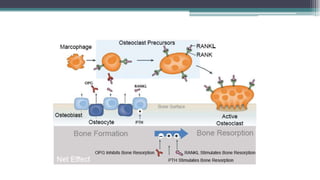
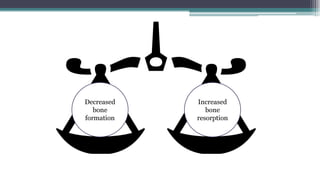
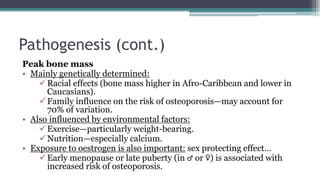
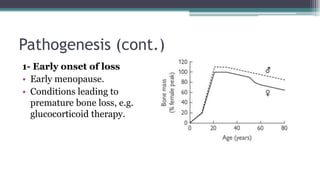
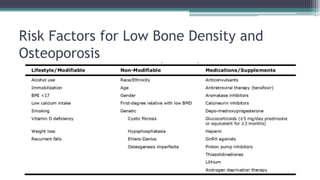
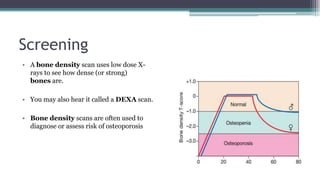
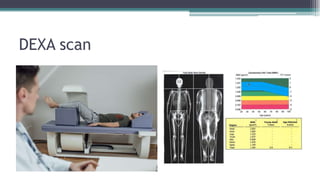
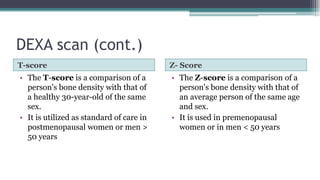
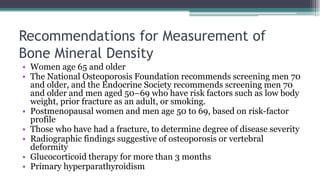
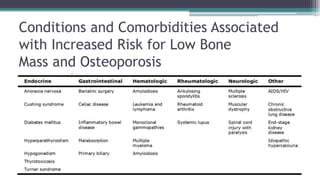
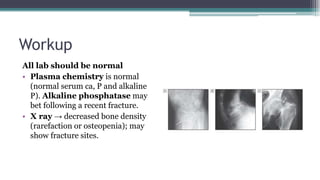
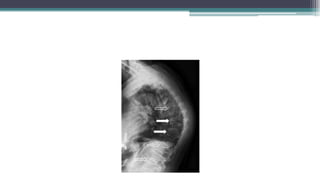
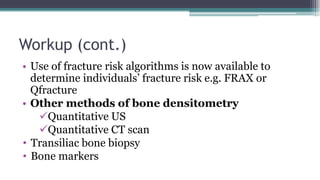
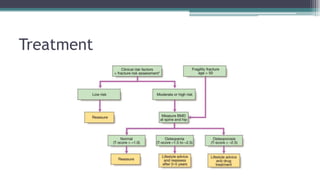
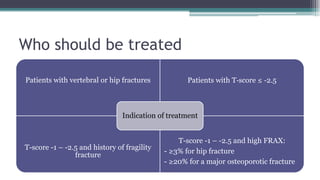
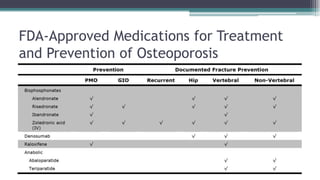
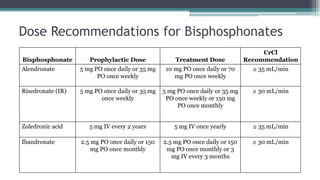
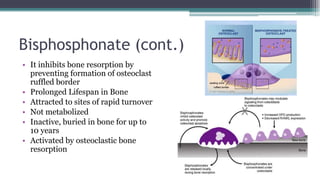
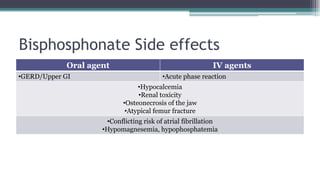
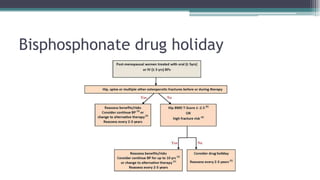
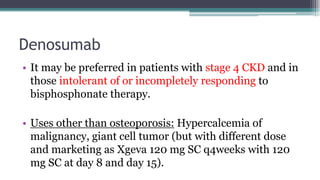
Metabolic bone diseases, such as osteoporosis and osteomalacia, result in bone abnormalities due to issues like calcium and phosphate imbalances. Osteomalacia is characterized by defective bone mineralization leading to pain, weakness, and fractures, largely stemming from vitamin D deficiency. Diagnosis involves lab tests for calcium and phosphorus levels, imaging for bone density assessment, and treatment focuses on correcting underlying causes and supplementing vitamin D and calcium.