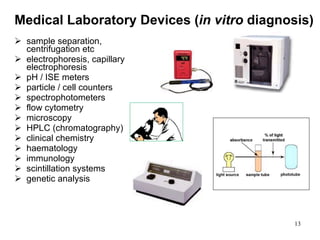
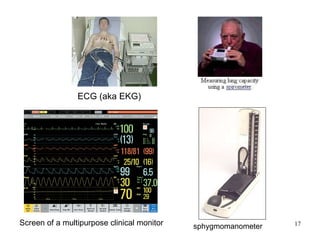
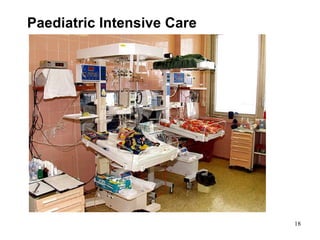
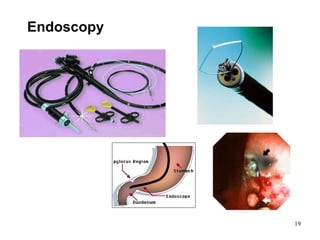
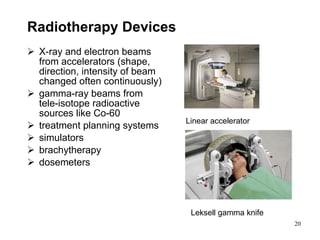
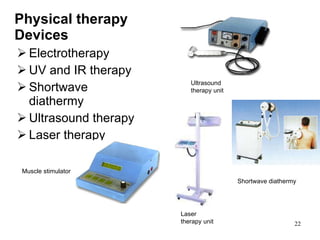
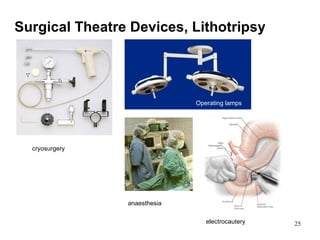
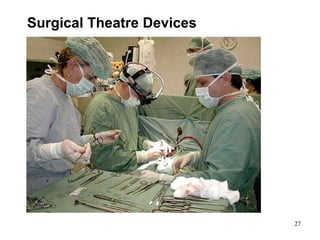
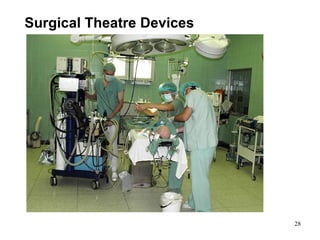
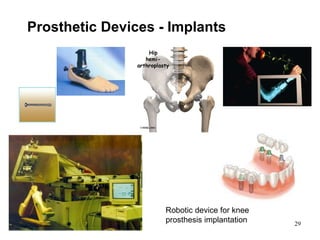
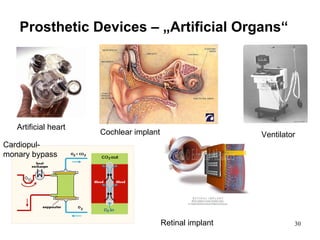
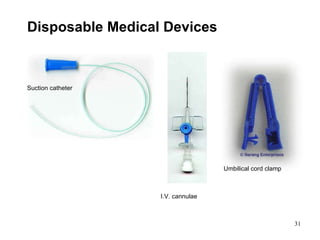
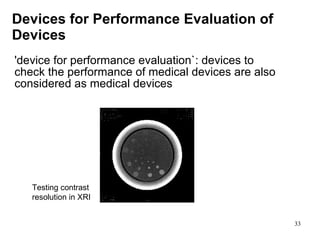
This document provides an overview of medical devices, including definitions, classifications, examples of different types of devices used for diagnosis, treatment and monitoring, and the theoretical principles underlying their functions. It discusses the purpose of medical biophysics education which is to ensure safe and effective use of devices through understanding their physics and applying protocols to minimize risks to patients and users. Competencies for medical device users are outlined, such as understanding device functions, limitations, quality control, and adhering to relevant regulations.