Embed presentation
Downloaded 12 times



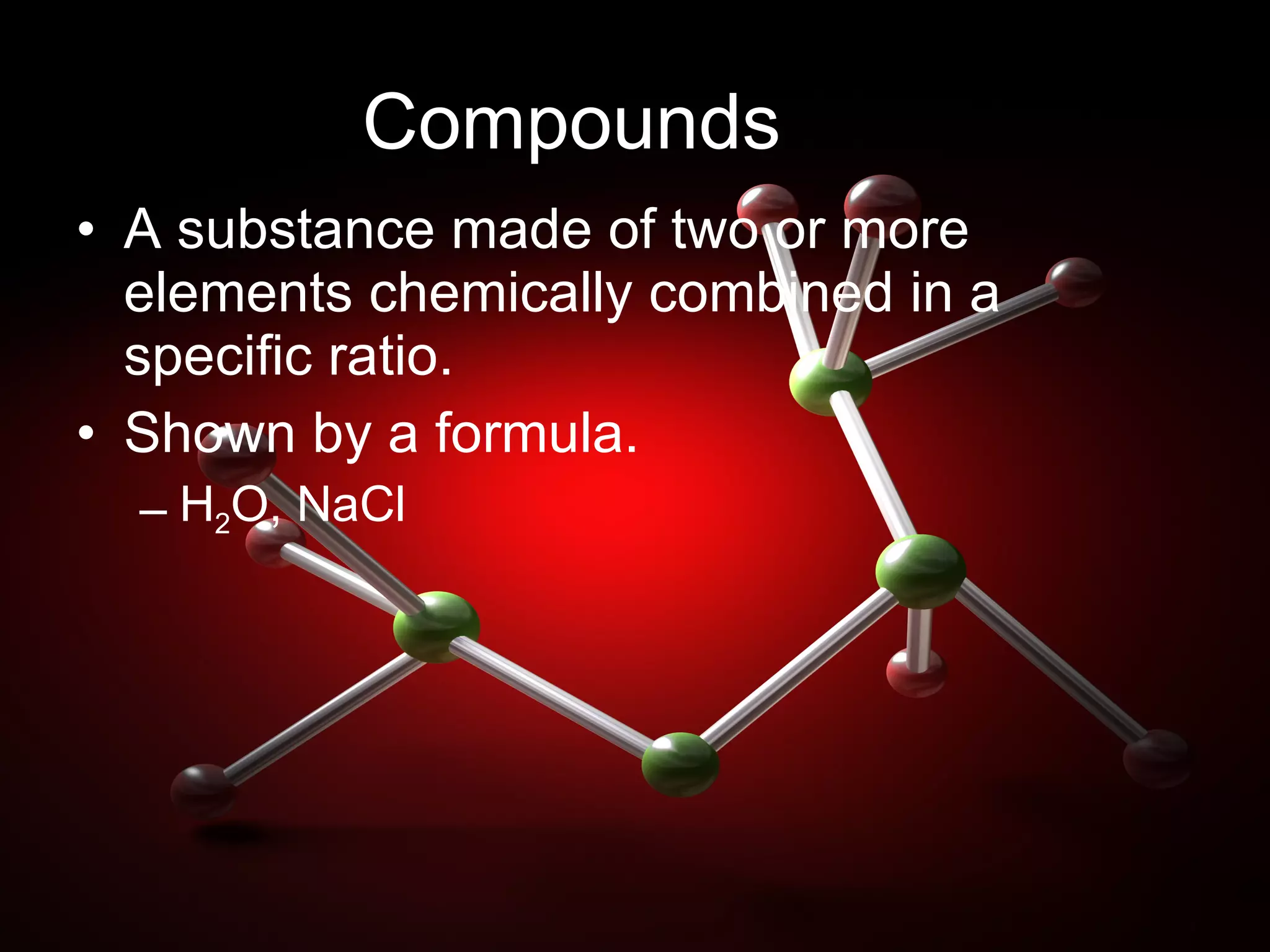



Matter is anything that takes up space and has mass, including invisible substances. It is made of elements, the basic building blocks of atoms, and compounds, which are combinations of two or more elements chemically bonded together. Matter can also exist as mixtures of elements and compounds mixed together without chemical bonds. Physical changes alter the state of matter between solid, liquid and gas, while chemical changes create new substances through chemical reactions.






