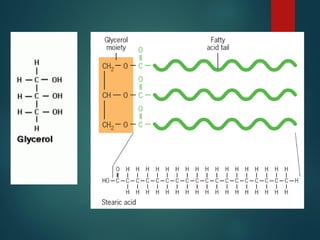
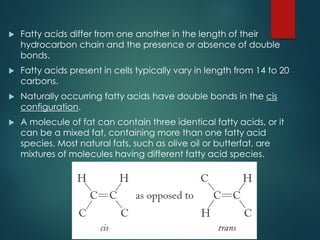
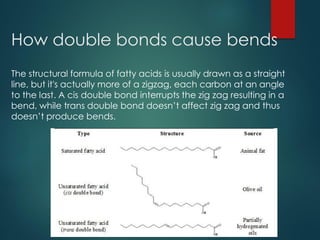
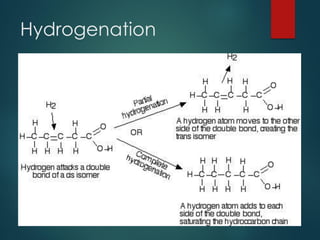
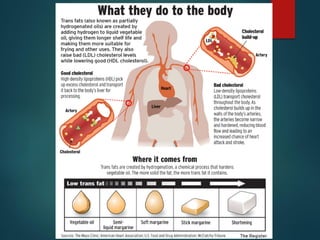
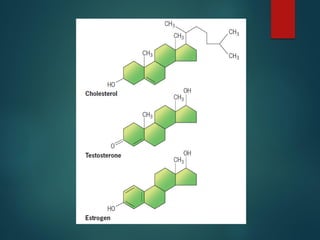
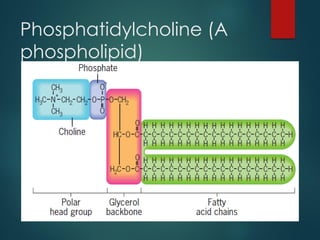
Lipids are nonpolar biological molecules important for cellular functions, including fats, steroids, and phospholipids. Fats, which are composed of glycerol and fatty acids, can vary in structure and include saturated and unsaturated types, with unsaturated fats containing double bonds that affect their physical properties. Phospholipids, essential for cell membranes, have distinct hydrophilic and hydrophobic regions due to their structure, which influences the properties of the membranes they comprise.