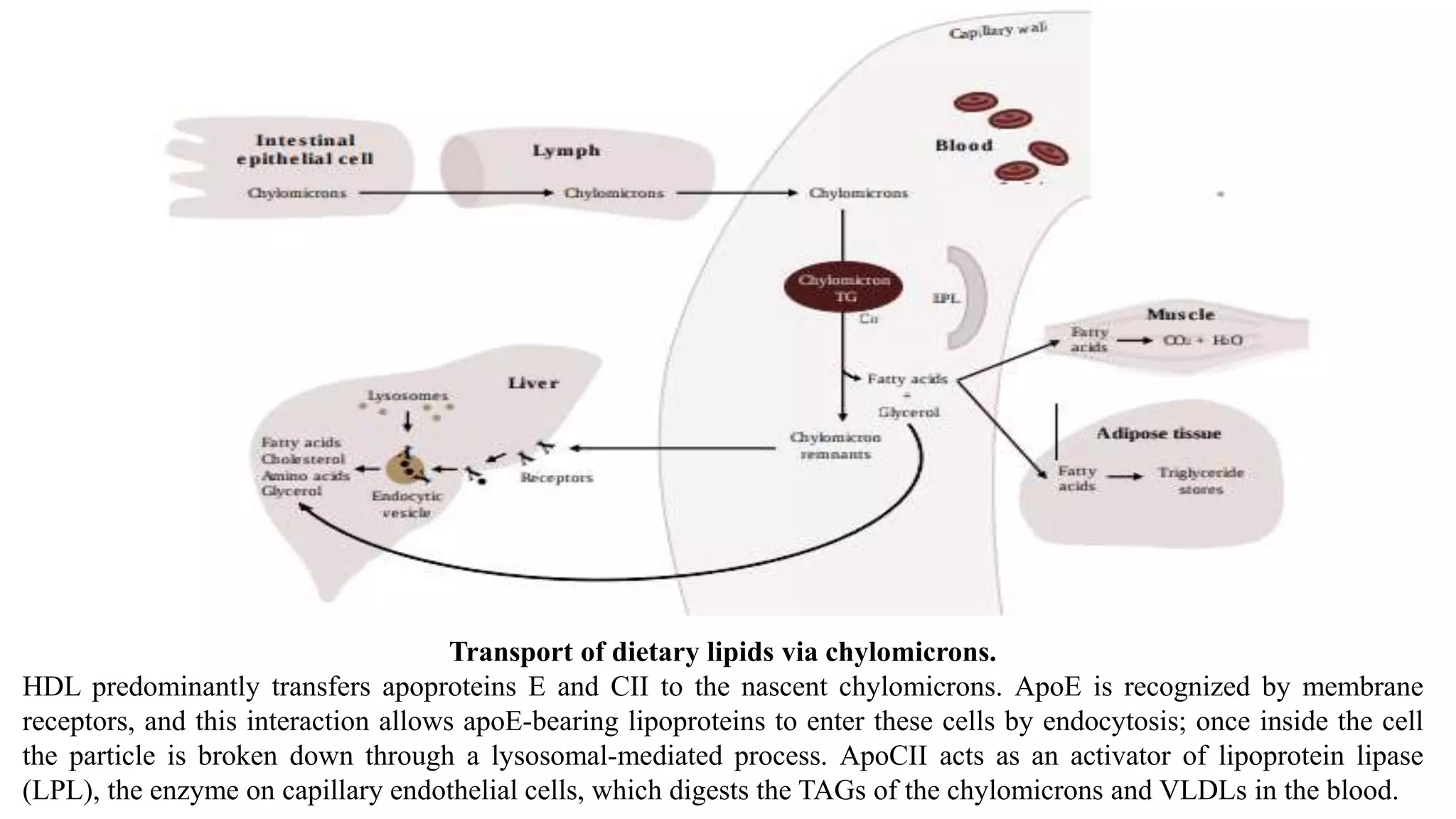
This document summarizes lipid transport via lipoproteins. It discusses the main classes of lipids transported in the body including fatty acids, triacylglycerols, and cholesterol. These lipids are insoluble in water and require transport via lipoproteins, which are complexes of lipids and proteins called apolipoproteins. The major classes of lipoproteins - chylomicrons, VLDL, IDL, LDL, and HDL - are described in detail regarding their structure, function, and role in lipid transport. Chylomicrons transport dietary lipids from the intestine to other tissues, while VLDL transports lipids synthesized in the liver. Apolipoproteins aid in lipoprotein structure and direct their metabolism.