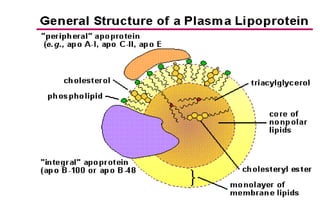
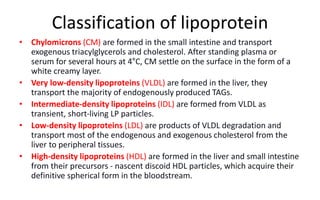
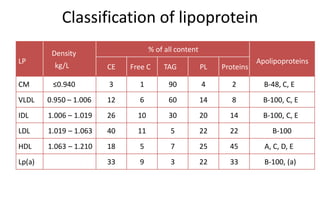
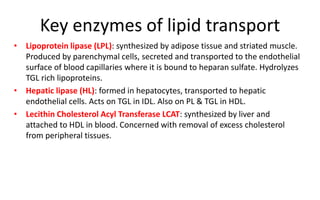
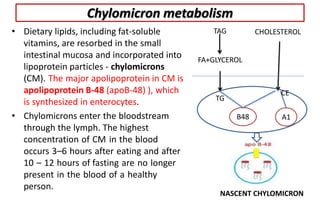
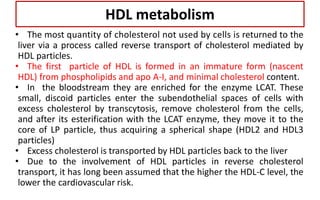
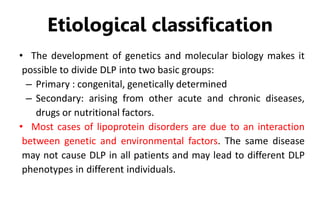
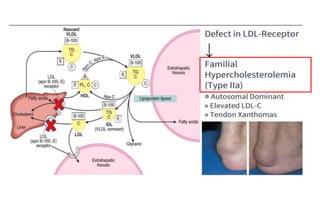
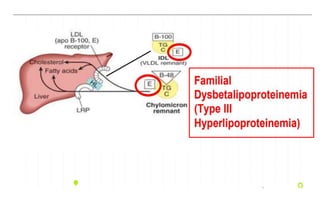
There are six major classes of lipoproteins in blood that are classified based on their density. Different analytical techniques separate lipoproteins based on properties like density, size, and electric charge. The major lipoproteins are chylomicrons, very low density lipoproteins (VLDL), intermediate density lipoproteins (IDL), low density lipoproteins (LDL), and high density lipoproteins (HDL). Apolipoproteins associated with each lipoprotein particle help maintain their structure and facilitate their metabolism and clearance from circulation.