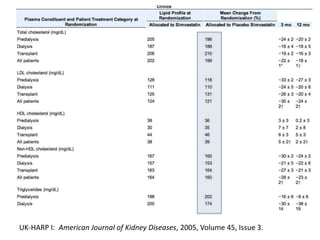
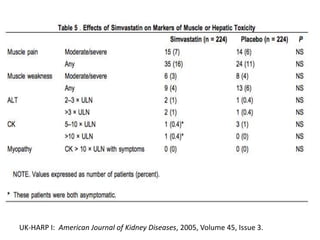
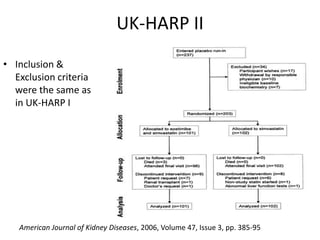
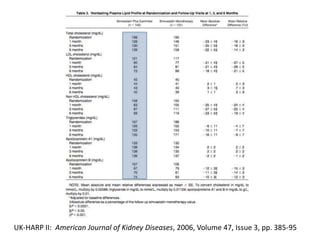
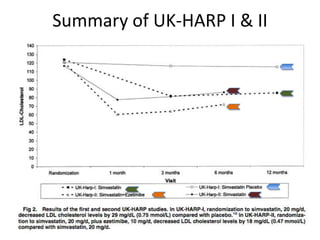
Lipid management in chronic kidney disease patients is controversial due to a lack of large randomized controlled trials. However, two pilot studies called UK-HARP I and II found that simvastatin alone or combined with ezetimibe lowered LDL and total cholesterol levels safely in CKD patients. This paved the way for the larger SHARP trial to determine if lipid-lowering agents can slow CKD progression and delay time to dialysis. Until results from SHARP are available, existing evidence suggests initiating statin therapy lowers risks of heart attack, death from heart disease, and need for heart procedures in CKD patients.