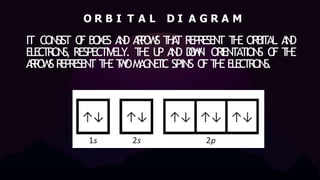
1. The document discusses electron configurations and orbital diagrams, which are two notations used to represent the distribution of electrons in different atomic orbitals. It explains the Aufbau principle, Pauli exclusion principle, and Hund's rule, which are general rules followed in representing electron distributions.
2. It provides an example problem of determining the electron configuration and drawing the orbital diagram for oxygen and its ion formed when oxygen gains two electrons. The solutions show the 1s2 2s2 2p4 electron configuration and orbital diagram for oxygen, and the 1s2 2s2 2p6 electron configuration and orbital diagram for the O2- ion.
3. It asks to determine the ground state electron configurations










![L
O
N
G E
L
E
C
T
R
O
N CONFIGURATIONS, ESPECIALLY T
H
O
S
E F
O
R E
L
E
M
E
N
T
S W
I
T
H
H
I
G
H A
T
O
M
I
C N
U
M
B
E
R
, C
A
N B
E C
O
N
V
E
N
I
E
N
T
L
Y S
H
O
R
T
E
N
E
D USING C
O
R
E
SYMBOLS. C
O
R
E SYMSBOLS, W
R
I
T
T
E
N A
S E
L
E
M
E
N
T S
Y
M
B
O
L INSIDE
B
R
A
C
K
E
T
S A
R
E R
E
P
R
E
S
E
N
T
A
T
I
O
N O
F T
H
E E
L
E
C
T
R
O
N C
O
N
F
I
G
U
R
A
T
I
O
N O
F T
H
E
N
O
B
L
E G
A
S T
H
A
T B
E
L
O
N
G T
O T
H
E R
O
WB
E
F
O
R
E T
H
A
T O
F T
H
E E
L
E
M
E
N
T IN
QUESTION.
F
O
R EXAMPLE, T
H
E C
O
N
F
I
G
U
R
A
T
I
O
N F
O
R N
E
O
N (Z=10) IS P
L
A
C
E
D IN A
B
R
A
C
K
E
T [NE]= 1S^2 2S^2 2P^6. S
O
D
I
U
M (Z=11), CITH C
O
N
F
I
G
U
R
A
T
I
O
N
1S^2 2S^2 2P^6 3S^1, M
A
YT
H
E
N R
E
P
R
E
S
E
N
T
E
D A
S [NE]3S^1.
N O B L E G A S E L E C T R O N
C O N F I G U R A T I O N](https://image.slidesharecdn.com/lesson2-240131121017-31a6cbfc/85/lesson-2-2-electron-distribution-pptx-12-320.jpg)

