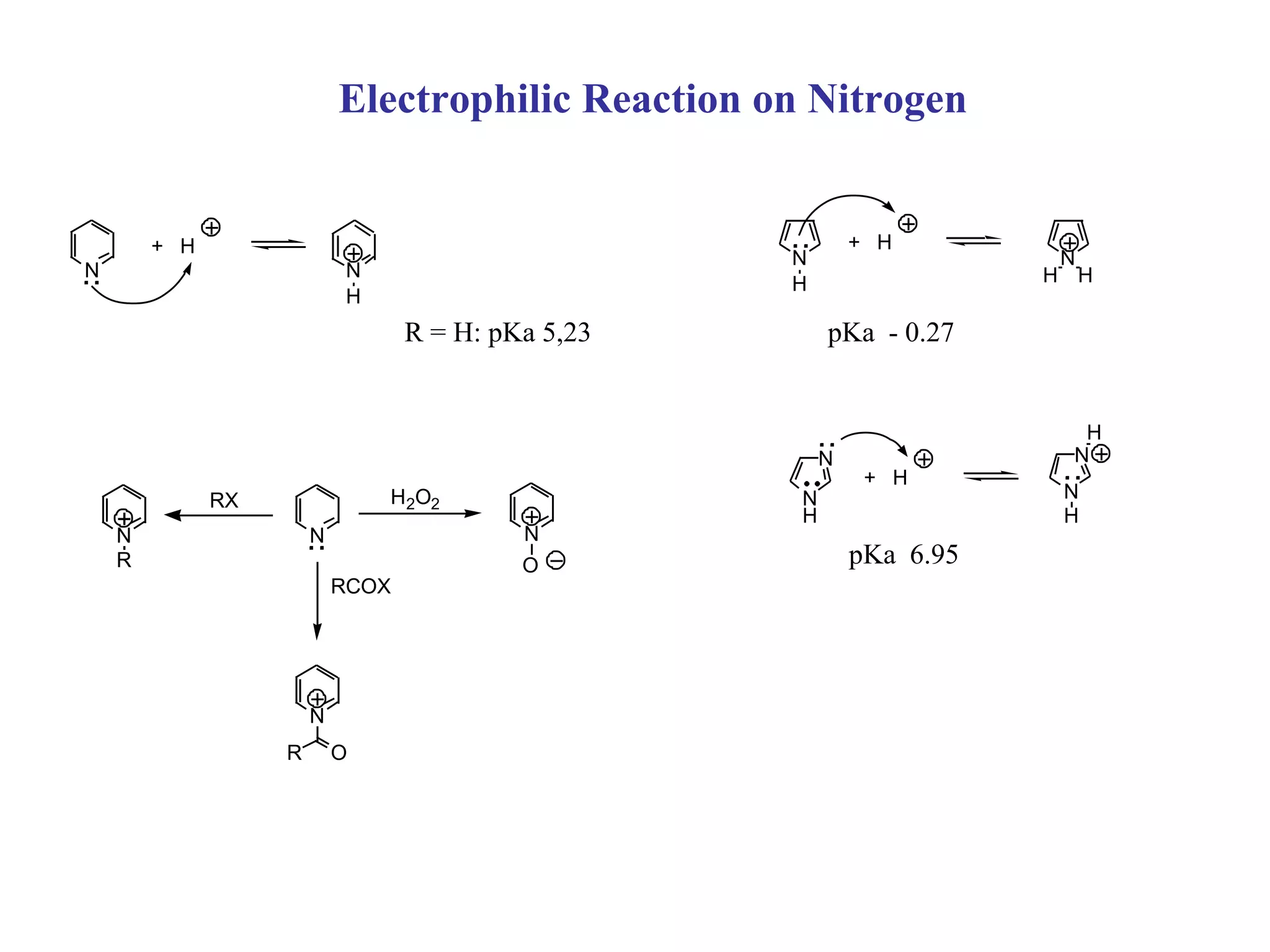
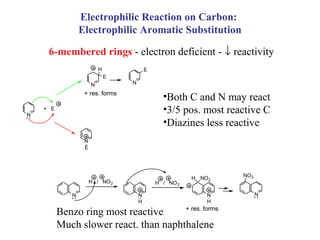
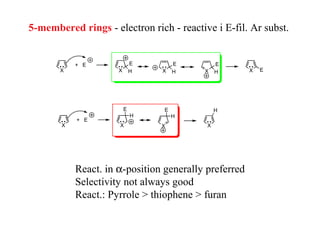
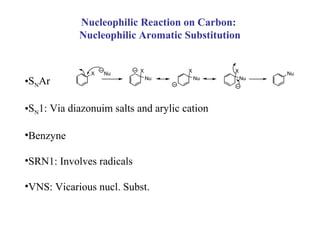
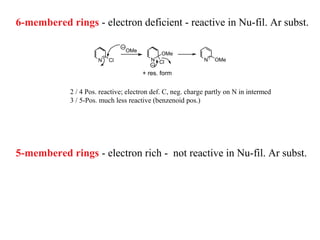
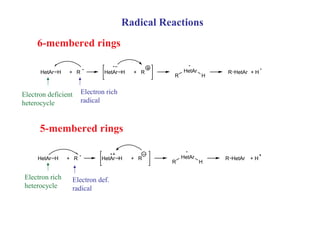
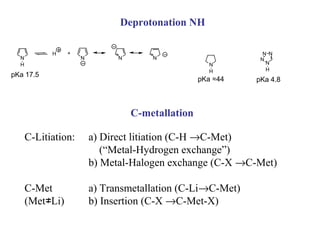
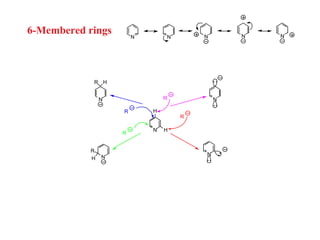
1) Electrophilic and nucleophilic aromatic substitution reactions can occur on both carbon and nitrogen atoms in heterocycles, with positions of reactivity depending on ring size and substitution.
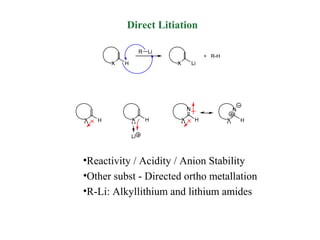
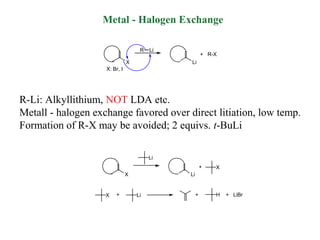
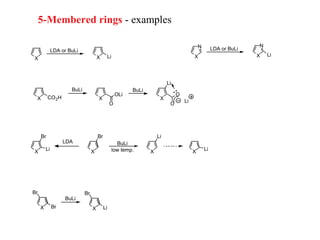
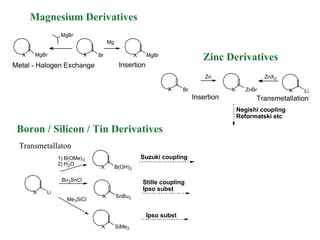
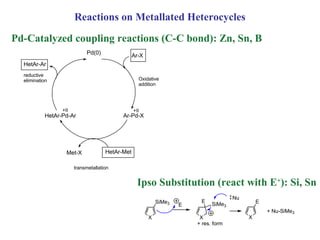
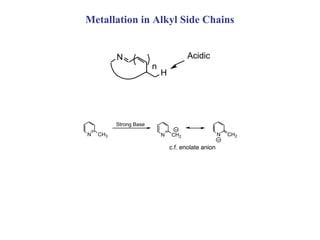
2) Directed metalation techniques like lithiation and Grignard reactions allow for functionalization of heterocycles at specific positions. Metal derivatives can then undergo cross-coupling, substitution, or other transformations.
3) Five-membered rings are generally more reactive than six-membered rings towards electrophiles due to their electron-rich nature. The reverse is true for nucleophilic aromatic substitution.