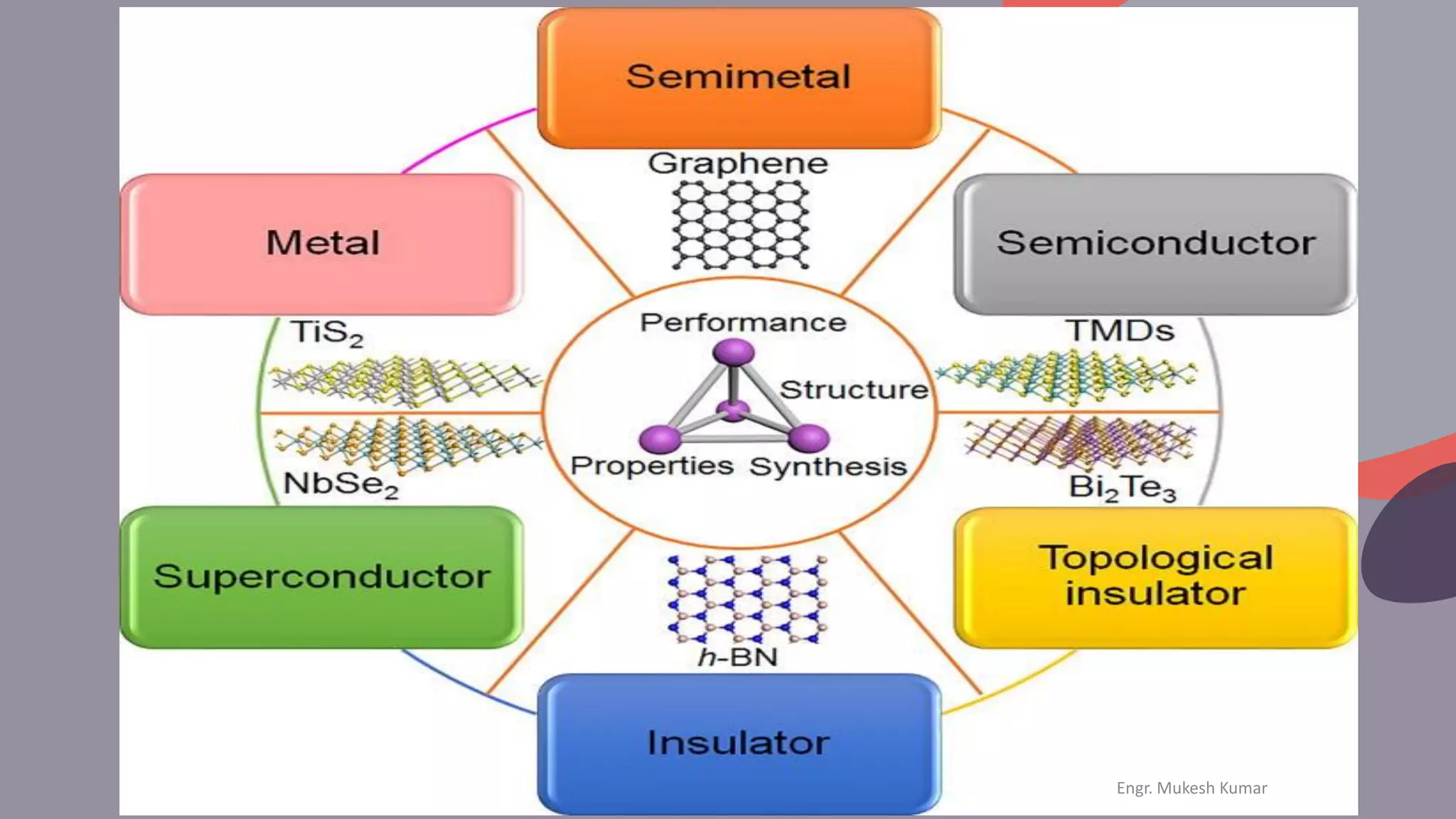
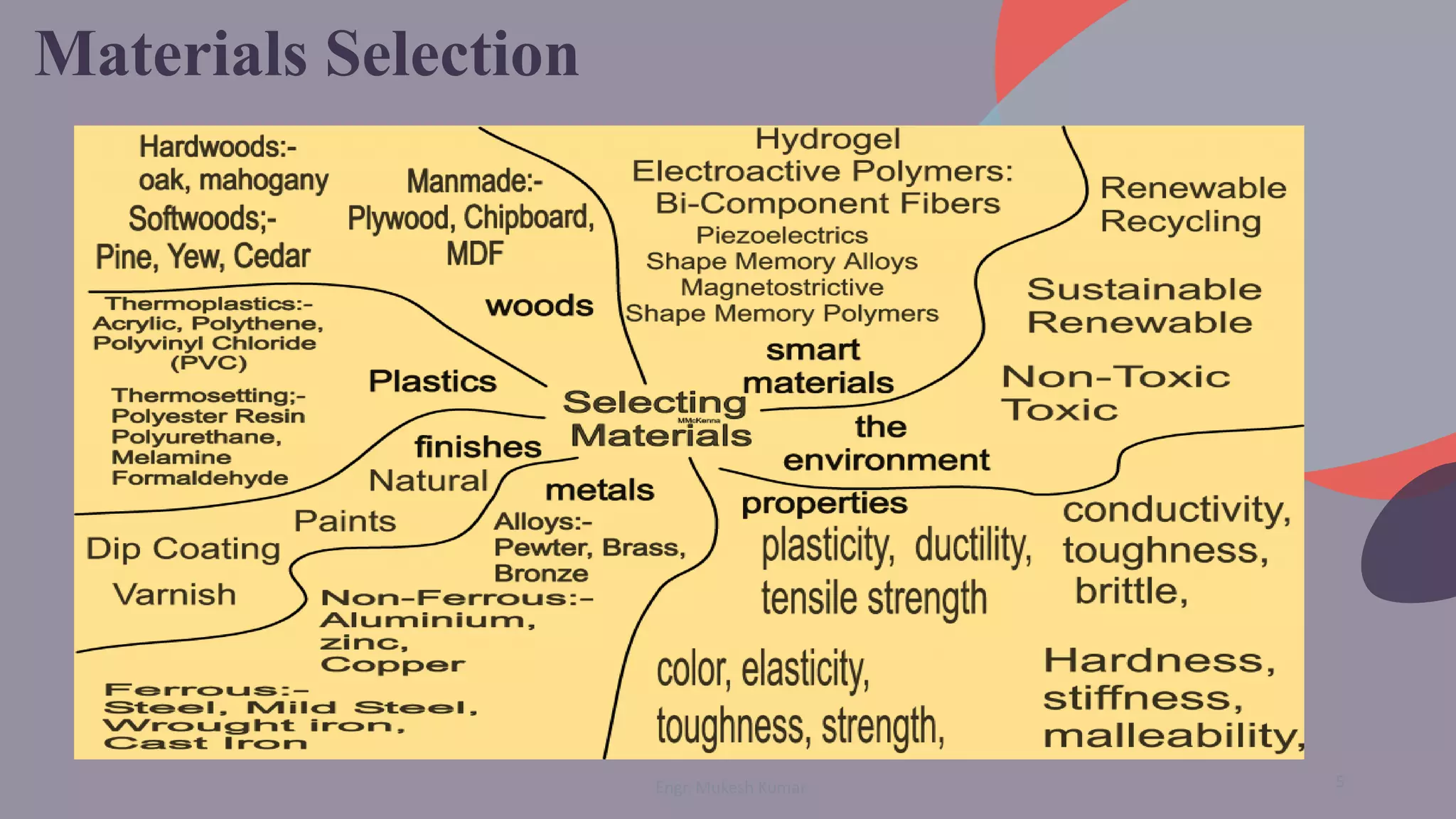
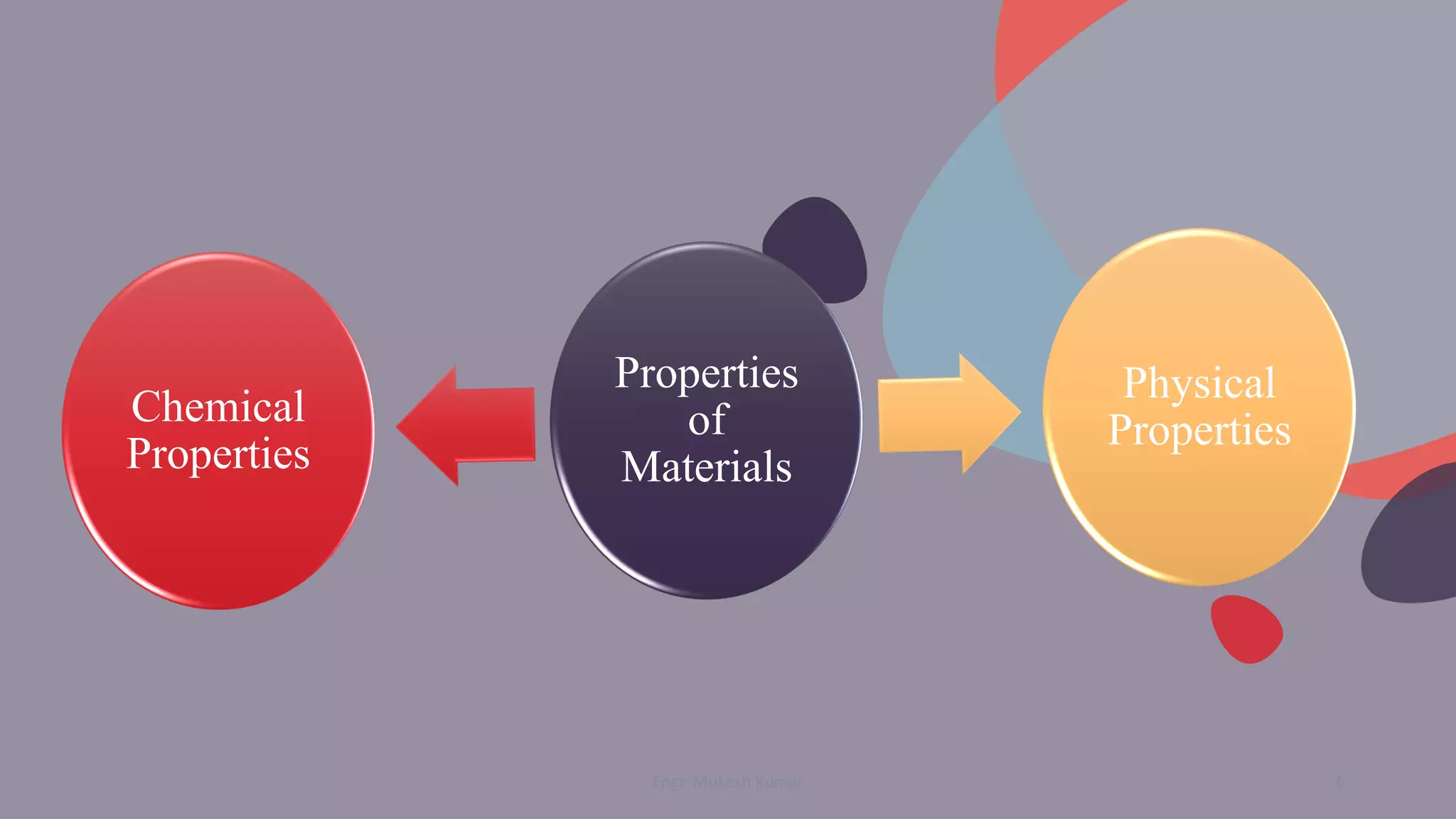
The document discusses the physical properties of materials, defining and classifying both physical and chemical properties. It emphasizes the importance of understanding these properties in material selection and engineering applications. Additionally, it poses questions to engage students in exploring the characteristics of various materials and their behaviors.