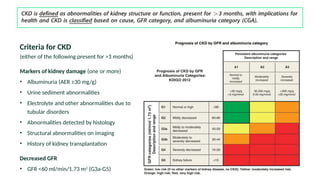
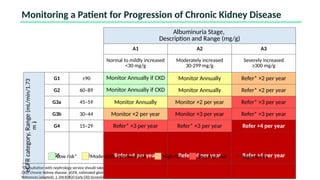
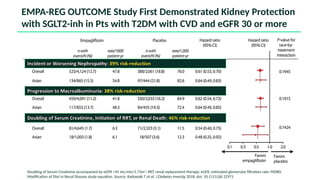
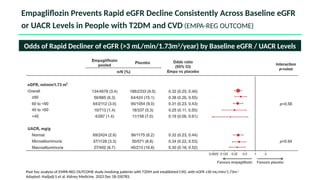
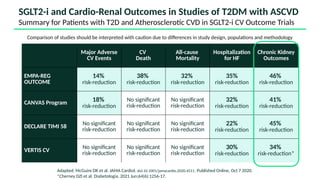
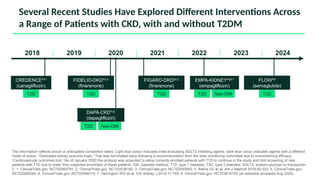
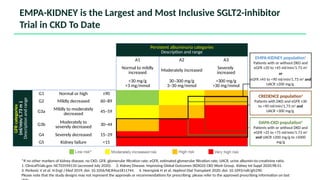
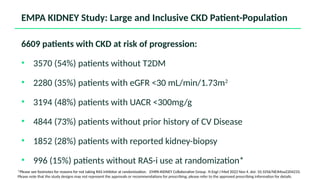
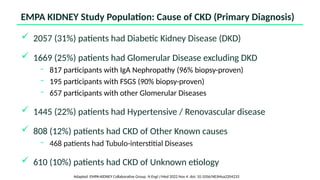
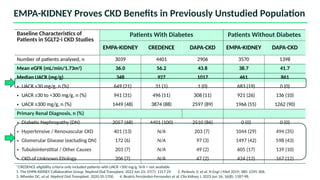
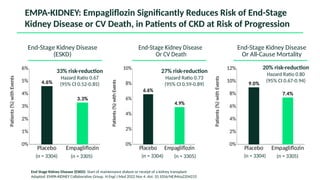
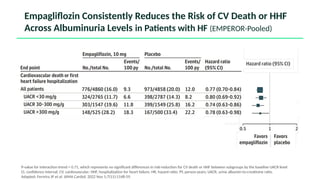
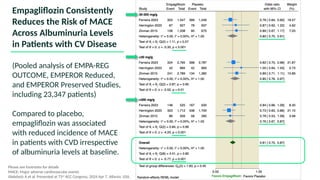
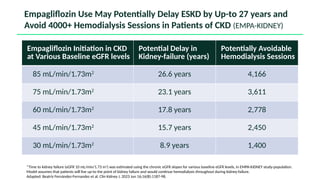
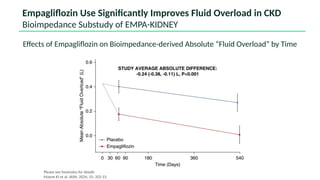
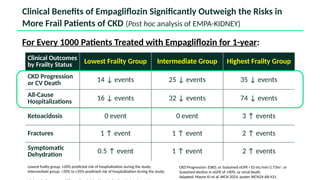
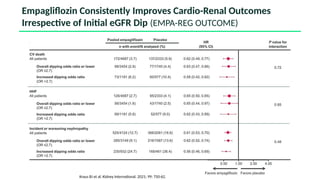
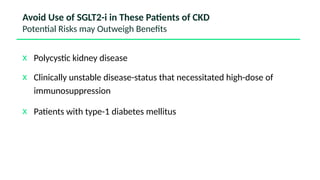
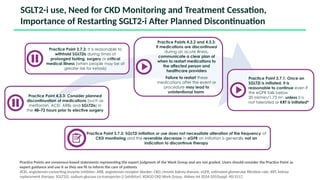
The document discusses the implementation of SGLT2 inhibitors in the management of chronic kidney disease (CKD), emphasizing the findings from the EMPA-KIDNEY study. It covers patient monitoring, the importance of nephrology consultation, and the trial design aimed at evaluating empagliflozin's efficacy in reducing kidney disease progression and cardiovascular death. Additionally, it details the study population's characteristics and various outcomes measured to assess the impact of SGLT2 inhibitors in CKD patients.