More Related Content
Similar to L7b Pressure drop, CSTR start up and semibatch reactors examples.pptx
Similar to L7b Pressure drop, CSTR start up and semibatch reactors examples.pptx (20)
L7b Pressure drop, CSTR start up and semibatch reactors examples.pptx
- 1. L7b-1
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
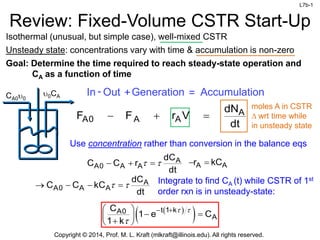
Review: Fixed-Volume CSTR Start-Up
Isothermal (unusual, but simple case), well-mixed CSTR
Unsteady state: concentrations vary with time & accumulation is non-zero
Goal: Determine the time required to reach steady-state operation and
CA as a function of time
moles A in CSTR
D wrt time while
in unsteady state
In Out
- +Generation = Accumulation
A
A0 A A
dN
F F r V
dt
CA0u0
u0CA
Use concentration rather than conversion in the balance eqs
t 1 k
A0
A
C
1 e C
1 k
A
A0 A A
dC
C C r
dt
A A
r kC
Integrate to find CA (t) while CSTR of 1st
order rxn is in unsteady-state:
A
A0 A A
dC
C C kC
dt
- 2. L7b-2
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
Review: Time to Reach Steady-State
t 1 k
A0
A
C
1 e C
1 k
At steady state,
t is large and: 0
A0
AS
C
C
1 k
In the unsteady state,
when CA = 0.99CAS:
t 1 k
A0 A0
s
C C
1 e 0.99
1 k 1 k
4.6 t
1 k
time to reach 99% (CA = 0.99CAS) of
steady-state concentration in terms of k
99% of the steady-state
concentration is achieved at: A AS
4.6 C 0.99C
1 k
When k is very small
(slow rxn), 1>>k: s
t 4.6
When k is very big
(fast rxn), 1<<k s
4.6
t
k
63% of the steady-state
concentration is achieved at: 1 k
CA = 0.63CAS
- 3. L7b-3
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
Semi-batch
FB
Review: Enhanced Yield in Semi-
Batch Reactor
V0 Vf
FD
V0 - u0t
Scenario 2: Improve the product yield obtained from a reversible reaction
A l B l C l D g
Allowing D(g) to bubble out of solution pushes equilibrium towards completion
A+B⇌
C+D
A+B
Scenario 1: Enhance selectivity of desired product over undesired side product
Higher concentrations of A favor formation of the desired product
Higher concentrations of B favor formation of the undesired side product
A
A+B
→P
V0 + u0t
V0 + u0t
Scenario 1 shown in blue. Scenario 2 shown in red.
- 4. L7b-4
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
Review: Mole Balance on A for
Semi-Batch Reactor
CBu0
V0 + u0t
In Out
- + Generation = Accumulation
A
A
dN
0 0 r V
dt
Use whatever units are most convenient (NA, CA, XA, etc)
A
A A A
N
C N C V
V
A
A
dC V
r V
dt
A
A A
dC dV
r V V C
dt dt
Convert NA to CA using:
In Out
- + Generation = Accumulation
Reactor volume balance:
0 0
d V
0 0
dt
u
u = u0
0
0
dV
dt
u
0 0
V t V
u
A
A A 0
dC
r V V C
dt
u
Rearrange to get in
terms of dCA/dt
A 0 A
A
C dC
r
V dt
u
Goal: Find how CA D with time (assume reactor is well-mixed)
- 5. L7b-5
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
Review: Mole Balance on B in
Semi-Batch Reactor
CBu0
V0 + u0t
Mole balance on B:
B
B B0
dN
r V F
dt
In Out
- + Generation = Accumulation
B
B0 B
dN
F 0 + r V
dt
0
dV
dt
u
B
B B B0 0 B B B0 0
dC
d dV
C V r V C C V r V C
dt dt dt
u u
0 B0 B
B
B
C C
dC
r
dt V
u
Substitute
Balance on B
B B
N C V
Rearrange to get in terms of dCB/dt
B
B 0 B B0 0
dC
C V r V C
dt
u u
Goal: Find how CB D with time (assume reactor is well-mixed)
- 6. L7b-6
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
Review: Semi-Batch Mole
Balances in Terms of NA
CBu0
V0 + u0t
A
A
dN
r V
dt
A
A
dN
0 0 r V
dt
In Out
- + Generation = Accumulation
0 0
N
V t V and C
V
u
A A A B
A
0 0
dN dN N N
r V k
dt dt V t
u
NB comes from basic mole balance:
B
A B0
dN
r V F
dt
B A B
B0
0 0
dN N N
k F
dt V t
u
The design eq in terms of XA can be messy. Sometimes it gives a single
equation when using Nj or Cj gives multiple reactor designs
A B
A 2
0 0
N N
then r k
V t
u
Substitute: -rA = kACACB and
Goal: Find how NA & NB D with time (reactor is well-mixed)
- 7. L7b-7
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X=0
P0 = 20 atm
PBR, 1000 kg cat
X=?
P = ? atm
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at 300
K, assume ideal gas behavior, and
the feed contains pure A (g).
2A→B -rA = kCA
2 α = 0.0008/kg
k=0.1 dm6/mol∙min∙kg cat at 300 K
FA0 = 10 mol min
A
A
A0
dX
F
d
r '
W
1. Mole balance
2. Rate law 2
A A
r kC
A0 A
A
A 0
C 1 X P
C
1 X P
3. Stoichiometry (put CA
in terms of X)
4. Combine
2 2
2
A0 A
0
A
A
0
2
A
C 1 X P
k
P
1 X
dX
dW F
2
2
A
A0
A
2
0 0
A
1 X
kC
dX P
dW P
1 X
u
5. Relate P/P0 to W
0
A
0 0
P
dP T
1 X
dW 2 T P P
1
- 8. L7b-8
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X=0
P0 = 20 atm
PBR, 1000 kg cat
X=?
P = ? atm
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at 300
K, assume ideal gas behavior, and
the feed contains pure A (g).
2A→B -rA = kCA
2 α = 0.0008/kg
k=0.1 dm6/mol∙min∙kg cat at 300 K
FA0 = 10 mol min
4. Combine
2
2
A
A0
A
2
0 0
A
1 X
kC
dX P
dW P
1 X
u
5. Relate P/P0 to W
0
A
0
P
dP
1 X
dW 2 P P
Simultaneously solve dXA/dW and dP/dW (or dy/dW) using Polymath
First, need to determine , CA0, & u0. Tf T0
T0
N N
N
1 2
0.5
2
What is CA0?
0
0 0 T0 0 A0
0
P
P V N RT C
RT
A0 3
3
20atm mol
C 0.813
dm
dm atm
0.082 300K
mol K
A0
0
A0
F
C
u
3
0
3
10mol min dm
12.3
min
0.813mol dm
u
- 9. L7b-10
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X=0
P0 = 20 atm
PBR, 1000 kg cat
X=?
P = ? atm
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at 300
K, assume ideal gas behavior, and
the feed contains pure A (g).
2A→B -rA = kCA
2 α = 0.0008/kg
k=0.1 dm6/mol∙min∙kg cat at 300 K
FA0 = 10 mol min
2
2
A
A0
A
2
0 0
A
1 X
kC
dX P
dW P
1 X
u
0
A
0
P
dP
1 X
dW 2 P P
Simultaneously solve dXA/dW and dP/dW (or dy/dW) using Polymath
0.5
A0 3
mol
C 0.813
dm
3
0
dm
12.3
min
u
- 10. L7b-11
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X=0
P0 = 20 atm
PBR, 1000 kg cat
X=?
P = ? atm
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at 300
K, assume ideal gas behavior, and
the feed contains pure A (g).
2A→B -rA = kCA
2 α = 0.0008/kg
k=0.1 dm6/mol∙min∙kg cat at 300 K
FA0 = 10 mol min
2
2
A
A0
A
2
0 0
A
1 X
kC
dX P
dW P
1 X
u
0
A
0
P
dP
1 X
dW 2 P P
Simultaneously solve dXA/dW and dP/dW (or dy/dW) using Polymath
0.5
A0 3
mol
C 0.813
dm
3
0
dm
12.3
min
u
- 11. L7b-12
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
X = 0.93
What conversion and P is
measured at the outlet of the
PBR? The rxn is isothermal at
300 K, assume ideal gas
behavior, and the feed contains
pure A (g).
P = 14.28 atm
- 12. L7b-13
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
A A
A0
dX r '
dW F
A0 A 0
A
A 0
C 1 X T
P
C
1 X P T
1
T T0
T0
N N 1 1
0
N 1
0
A0 A
A
F X
CSTR design eq: W
r'
Use info from PBR to determine FA0, CA0 & k
A A
A0
dX kC
dW F
A A0 A A A0 A
0
P 0.0008
C C 1 X C C 1 X 1 W
P kg
Isothermal and =0. Ergun eq for P/P0 becomes:
0
P
1 W
P
A
A
A0
A0
F
k
W
C
X
1 X
9atm
1 1000kg
20atm
0.2025 1 1000kg
1
0.0008 kg
Plug into CA:
Do not plug in P and P0 that occurred in PBR
yet! Use Ergun eq to get P/P0 as a function of
W, plug into design eq & integrate over W!
Use PBR expt
parameters to
solve for α
- 13. L7b-14
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
A0 A
A
F X
CSTR design eq: W
r'
Use info from PBR to determine FA0, CA0 & k
A A
A0
dX kC
dW F
A A0 A
0.0008
C C 1 X 1 W
kg
A
A
A0
A0
F
k
W
C
X
1 X
Plug CA into PBR
design eq:
A0
A
A
A0
kC
dX 0.0008
1 X 1 W
dW F kg
A0 A
A
A0
0.0008
k C 1 X 1 W
kg
dX
dW F
Rearrange
Integrate so that we can
get values of unknowns
- 14. L7b-15
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
A0 A
A
F X
CSTR design eq: W
r'
A
A
A0
A0
F
k
W
C
X
1 X
A
A
A
0
A0
kC
dX 0.0008
PBR design eq : 1 X 1 W
dW kg
F
A0
A
X W
A
A
A
0 0 0
dX 0.00
kC
F
08
1 W dW
1 X kg
1000k
A 2
0
A0
g
3
0
1 2 1 0.0008
ln 1 1 W
1 0.141 3 0.000
kC
F 8 kg kg
1000kg
3
2
A
0
A0
A0
1 2 0.0008
ln 1 1 W
1 X 3 0.0008
kC
F kg kg
- 15. L7b-16
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
A0 A
A
F X
CSTR design eq: W
r'
A
A
A0
A0
F
k
W
C
X
1 X
A
A
A
0
A0
kC
dX 0.0008
PBR design eq : 1 X 1 W
dW kg
F
A0
A0
1000kg
3
2
0
1 2 1 0.0008
ln 1 1 W
0.859 3 0.0008 kg kg
kC
F
A0
A0
kC
F
0.152 758.8kg
4 1 A0
A0
2.0 10 kg
kC
F
Plug this value into
the CSTR eq
A
3 3
2 2
0
A0
0.0008 0.0008
0.152 833.3 kg 1 1 1000kg 833.3 kg 1 1 0k
k
g
kg
C
F kg
A0
A
3
0
2
0.152 833.3 kg 1 1 0.8 833.3 kg 1 1
kC
F
A0
A0
0.152 833.3 kg 1 0.0894
kC
F
- 16. L7b-17
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
What conversion can be achieved in a
fluidized CSTR with the same catalyst weight
and P0 = P (ideal gas behavior, pure A feed)?
A0 A
A
F X
CSTR design eq: W
r'
A
A
A
A0
0
F
kC
X
W
1 X
4 1 A0
A0
kC
2.0 10 kg
F
4
A
A
1
1
2.0 10 k
X
1000kg
1 X
g
A
A A
A
X
0.2 0.2 0.2X X
1 X
A A
0.2 1.2X 0.17 X
Conversion in fluidized CSTR, no pressure drop
- 17. L7b-18
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
A + 2B → C Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
3
mol
k 53 at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
How many kg of catalyst is required to achieve XA = 0.8?
1. What is CA0? A0 A0 T0
C y C
A0
A0
T0
N
y =
N
T0
T0
N P
C
V RT
A0 A A0 0 A
A A
F X C X
W W
r' r'
u
Known: u0 and XA
Unknown: CA0 & -r’A
Fluidized
CSTR
design eq:
Feed is a stoichiometric mixture
→ 1 part A, 2 parts B A0
1 1
y =
1 2 3
A0 A0
P
C y
RT
A0 3
mol
C 0.055
dm
A0 3
1 6atm
C
3 atm dm
0.082 443K
mol K
- 18. L7b-19
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
A + 2B → C Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
How many kg of catalyst is required to achieve XA = 0.8?
2. What is –r’A?
A0 A A0 0 A
A A
F X C X
W W
r' r'
u
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
3
mol
Units on k are:
kg cat min atm
Express rate law in terms
of partial pressure, not Cj
A
2
B
A kP P
r '
3
mol
k 53 at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
2a. What is PA?
j
0
j
A
A
j j
C
C
X
1 X
For ideal, isobaric,
isothermal rxn:
i i
i
N P
C
V RT
Substitute for Cj & Cj0
j j A
A
j0
j
P
P RT
R
X
1 X
T
j0 j j A
j
A
P X
P
1 X
- 19. L7b-20
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
A + 2B → C Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
How many kg of catalyst is required to achieve XA = 0.8?
2. What is –r’A?
A0 A A0 0 A
A A
F X C X
W W
r' r'
u
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
Units on k necessitate expressing rate law in
terms of partial pressure, not Cj
3
mol
k 53 at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
j0 j j A
j
A
P X
P
1 X
2a. What is PA?
A=-1 A=1
j0 j0 0 j0 j0
J
A0 A0 0 A0 A0
F C C y
F C C y
u
u
T T0
A0
T0
N N
y
N
A0
1 2
y (1 2 1)
3 3
A0 A0 T0
P y P
A0 A0
1
P 6atm P 2atm
3
A
A
A
2atm 1 X
P
1 2 3 X
A
2
B
A kP P
r'
- 20. L7b-21
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
A + 2B → C Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
How many kg of catalyst is required to achieve XA = 0.8?
2. What is –r’A?
A0 A A0 0 A
A A
F X C X
W W
r' r'
u
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
3
mol
k 53 at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
2b. What is PB? B=-2
j0 j0 j0
J
A0 A0 A0
F C y
F C y
2
3
B0
B
A0
F 2
2
F 1
B
A0 j j A
A
P
P X
1 X
A
B
A
4atm 1 X
P
1 2 3 X
B
A
A
2atm 2 2X
1 2 3 X
P
A
A
A
2atm 1 X
P
1 2 3 X
A
2
B
A kP P
r'
- 21. L7b-22
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
A + 2B → C Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
How many kg of catalyst is required to achieve XA = 0.8?
2. What is –r’A?
A0 A A0 0 A
A A
F X C X
W W
r' r'
u
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
3
mol
k 53 at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
A
B
A
4atm 1 X
P
1 2 3 X
A
A
A
2atm 1 X
P
1 2 3 X
2c. What is k at 443K?
E 1 1
R T T
1 2
44 0
K 3
3 0 K
k e
k
80000J mol 1 1
8.314J mol K 300K 443K
3
443K
mol
53 e
kgcat min atm
k
6
443K 3
mol
k 1.663 10
kgcat min atm
A
2
B
A kP P
r'
- 22. L7b-23
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
2. What is –r’A?
A0 A A0 0 A
A A
F X C X
W W
r' r'
u
Known: u0, XA, & CA0 (0.055 mol/dm3)
Unknown: -r’A
A
2
B
A kP P
r '
A
B
A
4atm 1 X
P
1 2 3 X
A
A
A
2atm 1 X
P
1 2 3 X
6
443K 3
mol
k 1.663 10
kgcat min atm
A
A
3
2
A
A
A
6 2a 4a
mol
1.663 10
kgcat min at
tm 1 X
1 2 3
tm 1 X
1 2 3 X X
m
r '
3
6 3 A
A 3 3
A
1 X
mol
r' 1.663 10 32atm
kgcat min atm 1 2 3 X
A + 2B → C Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min
How many kg of catalyst is required to achieve XA = 0.8?
3
mol
k 53 at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
- 23. L7b-24
Copyright © 2014, Prof. M. L. Kraft (mlkraft@illinois.edu). All rights reserved.
How many kg of catalyst is required to achieve XA = 0.8?
3
mol
k 53 at 300K with E=80 kJ/mol, elementary rxn
kg cat min atm
3
3
3
6 3
3 3
1 0.8
mol
1.663 10 32atm
k
dm
50
gcat min atm 1 2 3 0.8
mol
0.055
d
W
8
mi
0.
m n
3
6 3 A
A 3 3
A
1 X
mol
r' 1.663 10 32atm
kgcat min atm 1 2 3 X
A0 0
A
A
C X
W
r '
u
CA0 =0.055 mol/dm3
7
W 5.24 10 kg cat
A + 2B → C Elementary rxn, feed is a stoichiometric mixture
Fluidized CSTR, isothermal, isobaric, ideal, gas-phase reaction
P0= 6 atm; T = 443K & u0 = 50 dm3/min