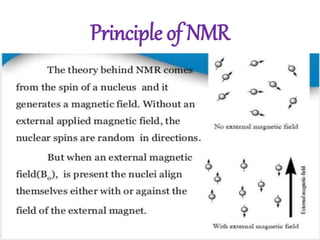
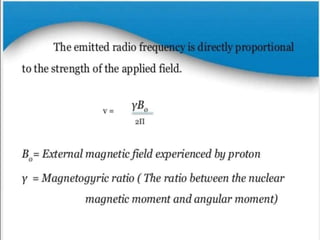
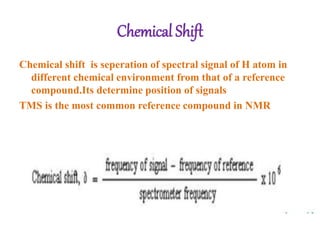
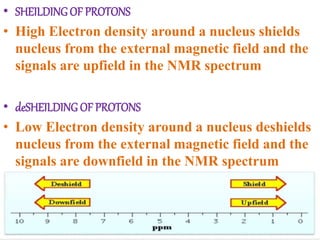
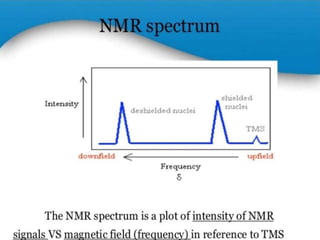
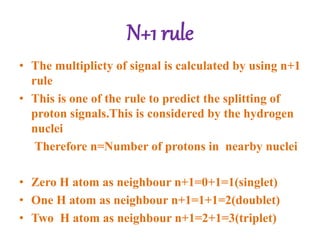
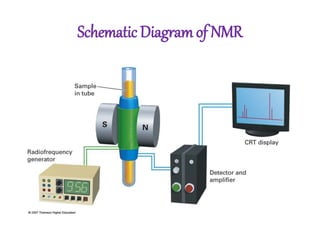
Nuclear magnetic resonance (NMR) spectroscopy uses radio waves to determine molecular structure by analyzing the magnetic properties of atomic nuclei. It works by placing a sample in a strong magnetic field, which causes the magnetic nuclei in the sample to absorb and emit radio signals. Analyzing these signals provides information on the molecular structure, such as identifying carbon-hydrogen frameworks in organic molecules. NMR is used in fields like organic chemistry, biochemistry, and medical research to study molecular structure and interactions.