This document contains sample questions from an IIT-JEE Advanced chemistry exam. It includes 12 questions on topics like oxidation-reduction reactions, isomerism, hydrogen bonding, electrochemistry, transition metals, and solutions. The total number of reagents that can oxidize iodide to iodine is 7. The molality of a 3.2 molar solution of a compound with molar mass 80 g/mol dissolved in a solvent with density 0.4 g/mL is 8.

![INORGANIC
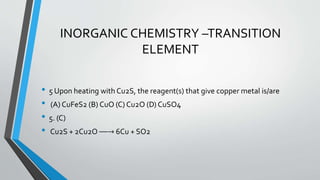
• 1.QUESTION. Consider the following list of reagents:
• Acidified K2Cr2O7, alkaline KMnO4, CuSO4, H2O2, CI2, O3, FeCI3, HNO3 and
Na2S2O3.
• The total number of reagents that can oxidise aqueous iodide to iodine is
• ANS. [7]
• H+
• / K2Cr2O7 , OH / KMnO4 , CuSO4 , H2O2 , Cl2 , O3, HNO3](https://image.slidesharecdn.com/jeemantra-141207235620-conversion-gate01/85/Jee-mantra-iit-jee-advanced-chemistry-paper-and-solution-2-320.jpg)






![9. A list of species having the formula XZ4 is given below.
• XeF4, SF4, SiF4, BF4−, [Cu(NH3)4]2+, [FeCI4]2−, [CoCl4]2− and [PtCI4]2−.
• Defining shape on the basis of the location of X and Z atoms, the total
number of species
• having a square planar shape is
• 9 [4]
• XeF4 , BrF4− , [Cu(NH3)4]+2 , [PtCl4]2−](https://image.slidesharecdn.com/jeemantra-141207235620-conversion-gate01/85/Jee-mantra-iit-jee-advanced-chemistry-paper-and-solution-10-320.jpg)
![inorganic
• 10. Among PbS, CuS, HgS, MnS, Ag2S, NiS, CoS, Bi2S3 and SnS2, the total
number of
• BLACK coloured sulphides is
• 10. [7]
• PbS , CuS, HgS, Ag2S, NiS, CoS, Bi2S3](https://image.slidesharecdn.com/jeemantra-141207235620-conversion-gate01/85/Jee-mantra-iit-jee-advanced-chemistry-paper-and-solution-11-320.jpg)
![inorganic
• 11. Consider the following list of reagents:
• Acidified K2Cr2O7, alkaline KMnO4, CuSO4, H2O2, CI2, O3, FeCI3, HNO3 and
Na2S2O3.
• The total number of reagents that can oxidise aqueous iodide to iodine is
• 11. [7]
• H+
• / K2Cr2O7 , OH / KMnO4 , CuSO4 , H2O2 , Cl2 , O3, HNO](https://image.slidesharecdn.com/jeemantra-141207235620-conversion-gate01/85/Jee-mantra-iit-jee-advanced-chemistry-paper-and-solution-12-320.jpg)
![Physical chemistry -solution
• 12. A compound H2X with molar weight of 80 g is dissolved in a solvent having density of
• 0.4 g ml−1
• . Assuming no change in volume upon dissolution, the molality of a 3.2 molar
• solution is
• 12. [8]
• Let us consider, volume of solution = 1 litre = 1000 mL
• wt. of solvent = 0.4 × 1000 = 400 g = 0.4 kg
• wt. of solute = 80 g ; no. of moles of solute = 3.2 moles
• molality of solution = 3.2](https://image.slidesharecdn.com/jeemantra-141207235620-conversion-gate01/85/Jee-mantra-iit-jee-advanced-chemistry-paper-and-solution-13-320.jpg)