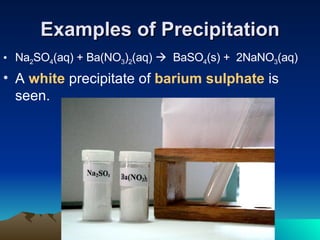
The document details two types of chemical reactions: neutralisation (acid-base reactions) and precipitation, suitable for upper secondary students. It explains neutralisation through the interaction of acids and bases leading to the formation of water, and provides examples, including reactions with different compounds. Additionally, it discusses precipitation reactions that produce insoluble compounds from soluble ones, highlighting examples involving silver and barium salts.