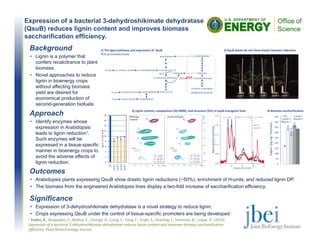
The document describes a study that screened 168 yeast strains for tolerance to the ionic liquid 1-ethyl-3-methylimidazolium acetate ([C2Ciim][OAc]). 13 strains were found to be tolerant to 5% [C2Ciim][OAc], with Galactomyces geotrichum being the most tolerant. G. geotrichum exhibited enhanced growth in the ionic liquid medium. Several yeasts were also identified that were capable of rapid growth and high cell density in 5% of the ionic liquid.
![Yeast tolerance to the ionic liquid 1-ethyl-
3-methylimidazolium acetate
Outcomes
• 13 yeast strains tolerant to 5% [C2Ciim][OAc] were identified.
• Galactomyces geotrichum was the most tolerant yeast. This yeast actually exhibited enhanced growth in IL medium.1
Several yeasts capable of rapid growth and high cell density in 5% IL were identified.
1Sitepu, et al., “Yeast tolerance to the ionic liquid 1-ethyl-3-methylimidazolium acetate.” FEMS Yeast Research
(in press)
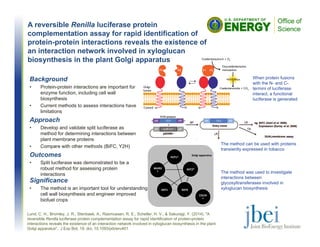
Background
• Pretreatment of biomass with
ionic liquids (ILs) can
improve hydrolysis. However,
residual IL on pretreated
biomass can inhibit many
fermentative
microorganisms.
• There is a need for yeasts
that can tolerate up to 4% of
the IL 1-ethyl-3-
methylimidazolium acetate
([C2Ciim][OAc]), a promsing
IL for pretreatment.
Significance
• These [C2Ciim][OAc]-tolerant yeasts could be used for fermentation of hydrolysates
from IL-pretreated biomass or serve as a source of yeast IL-tolerance pathways.
Finalcultureabsorbanceat600nm
Approach
• 168 strains of yeasts spanning multiple phyla from the Phaff Yeast Culture Collection at UC Davis were screened by
culturing in media containing varying levels of [C2Ciim][OAc].](https://image.slidesharecdn.com/jbeihighlightsoctober2014-161017203800/85/JBEI-Highlights-October-2014-1-320.jpg)