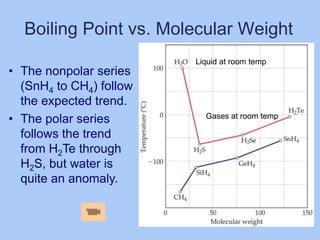
This document provides information for a chemistry class. It lists the topics to be covered, which include gases, liquids, solids and intermolecular forces from chapters 11.1-11.2. Students are assigned to work in groups and given reading assignments. The professor outlines the homework due for the next class and lab safety information. Office hours are provided, and a pre-class reading assignment is listed along with sample exercises to be completed.