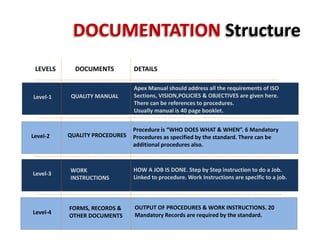
This document outlines the structure and requirements for documentation in an ISO 9001:2008 quality management system. It describes four levels of documentation including a quality manual, procedures, work instructions, and records/forms. The six mandatory procedures are also listed. Key requirements for procedures, work instructions, and the 20 mandatory records specified by the standard are provided. Guidance is given on document control including versioning, approvals, and distribution. Contact information is provided for assistance with ISO documentation and implementation needs.