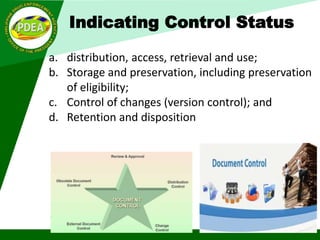
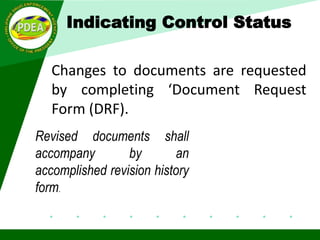
This document discusses ISO 9001:2015 requirements for quality management system documentation. It covers the purpose and benefits of a quality management system. The key requirements for quality system documentation include establishing, documenting, implementing, maintaining and improving the effectiveness of the quality system. The core documentation includes a quality manual, quality policy, objectives, procedures, processes and records. The document also discusses controlling documented information, including approval, identification, format, changes and retention. It provides examples of document structure, layout and control status indicators.