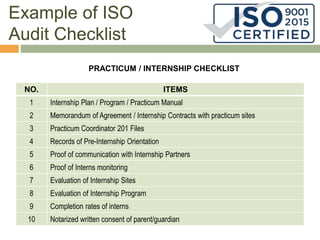
This document discusses ISO 9000 standards for quality management systems. It provides an overview of ISO standards and ISO 9000, including the principles of quality management. It then outlines the 8 steps for registration and certification, including finding a registrar, completing assessments, and surveillance audits. Finally, it gives an example ISO audit checklist for evaluating a university's quality management system regarding faculty, practicum/internships, and other areas.