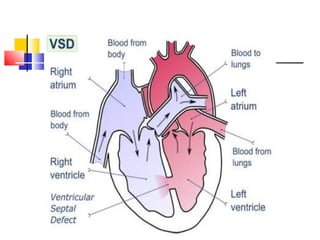
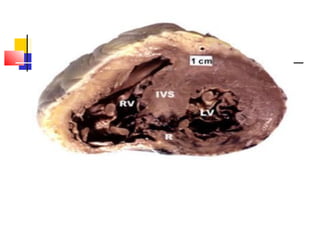
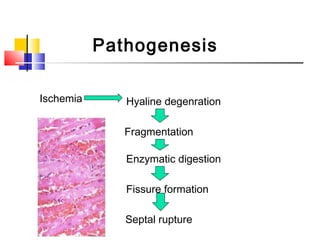
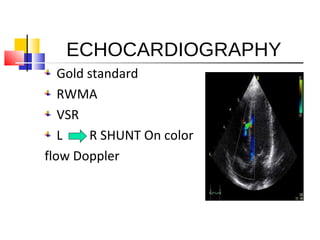
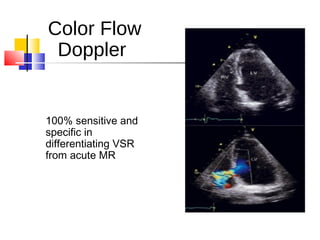
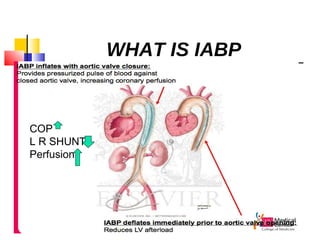
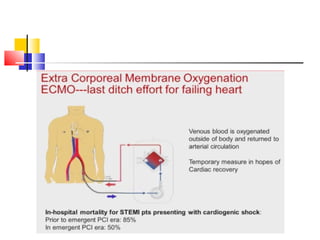
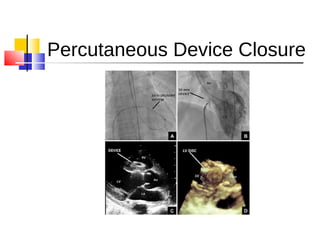
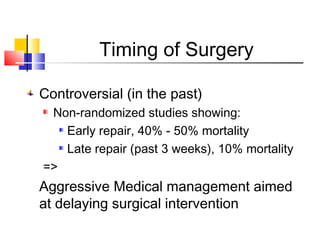
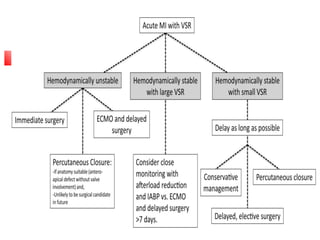
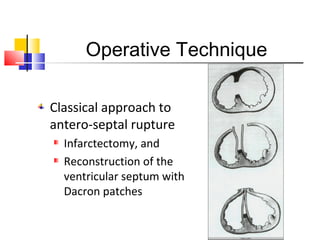
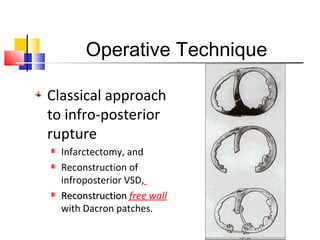
Ventricular septal rupture (VSR) is a rare but serious complication following myocardial infarction, with high mortality rates that have improved only slightly with early diagnosis and advanced treatment strategies. The pathophysiology involves ischemic damage, and risk factors include advanced age, female sex, and certain heart conditions, with diagnosis relying heavily on imaging methods like echocardiography. Treatment ranges from medical management to surgical repair, with timing being crucial for a successful outcome, especially in cases of cardiogenic shock.