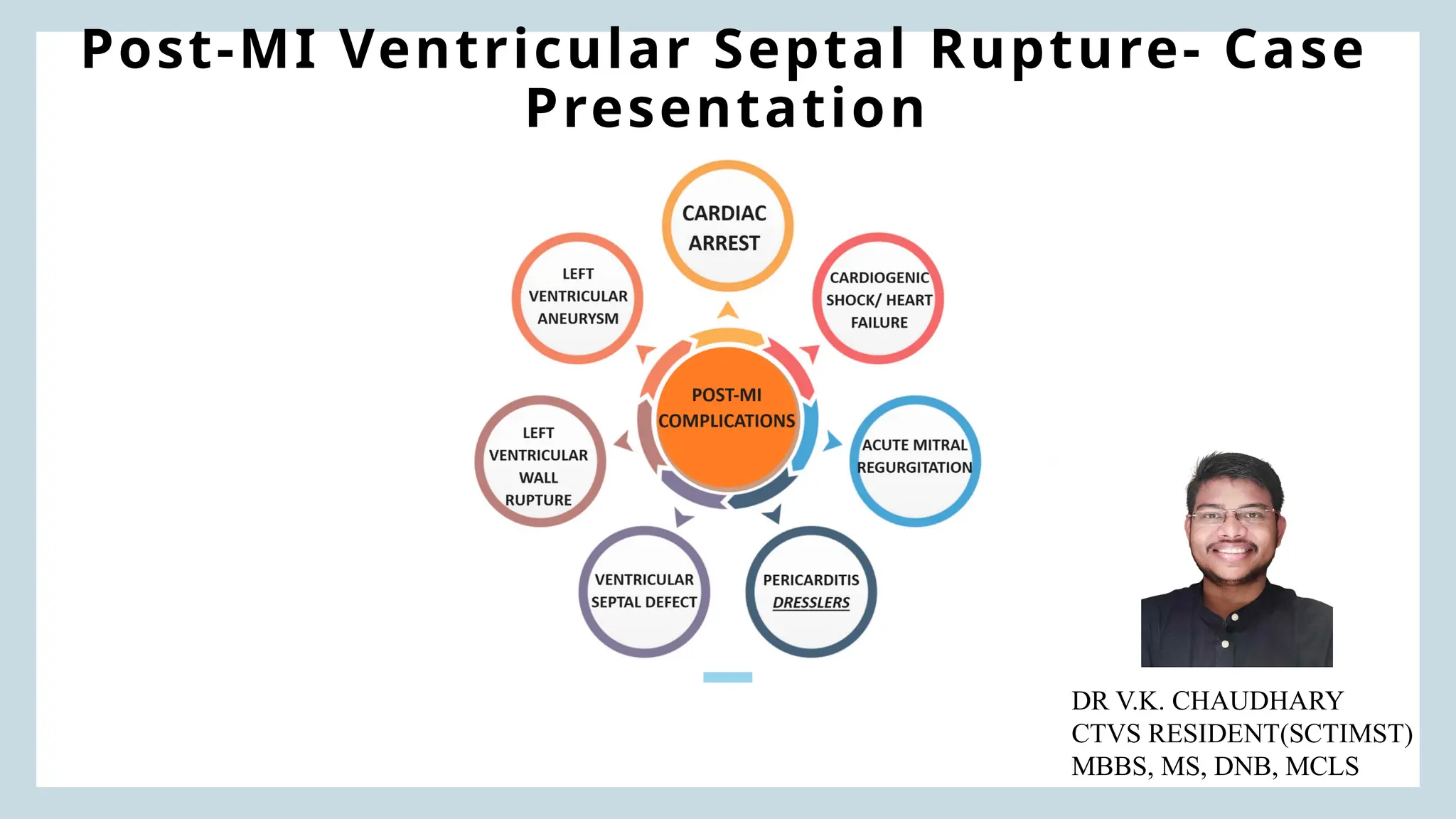
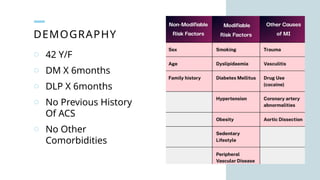
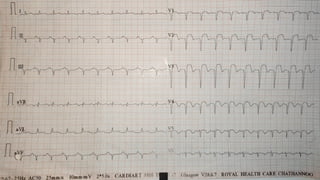
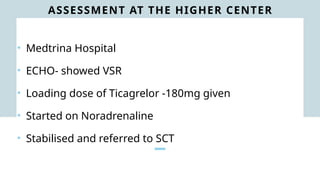
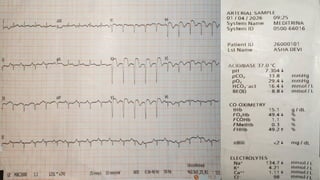
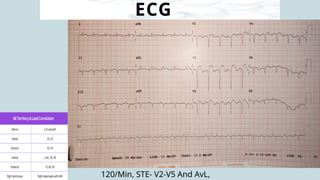
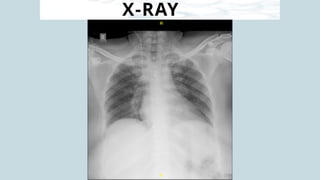
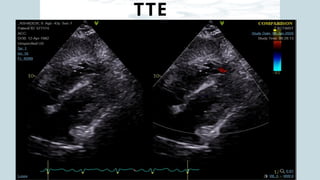
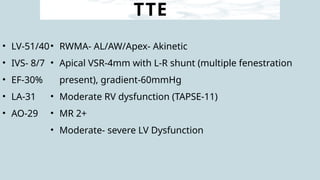
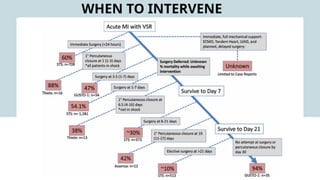
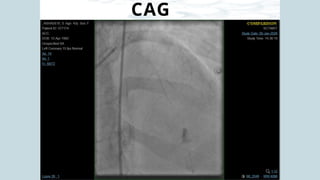
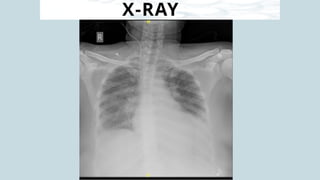
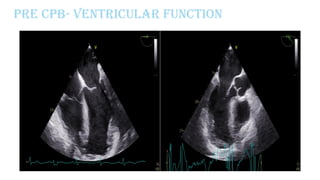
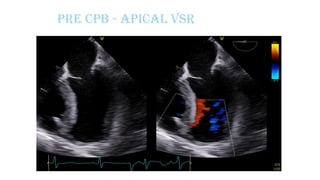
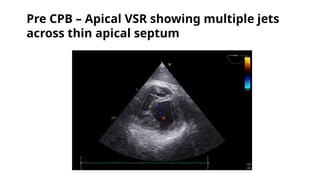
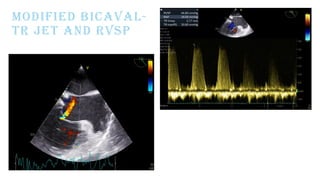
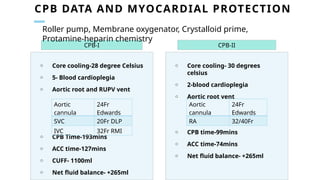
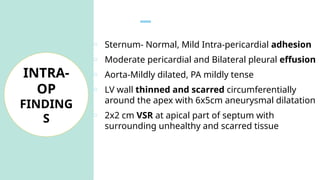
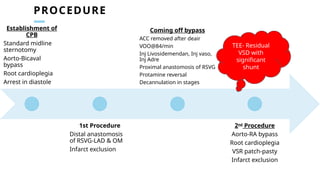
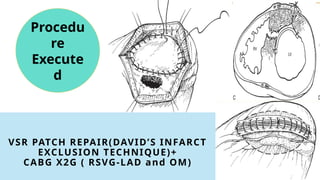
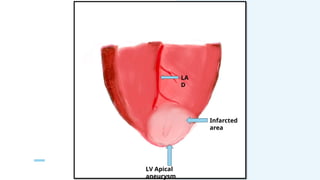
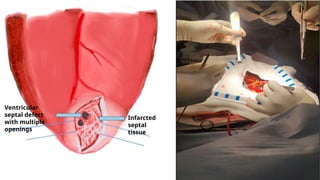
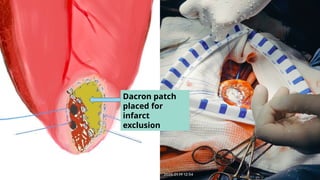
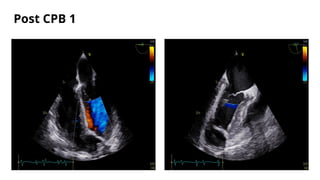
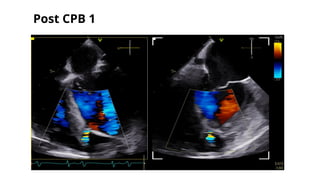
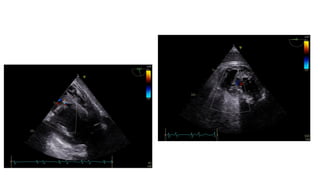
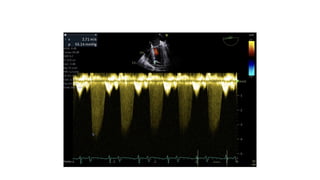
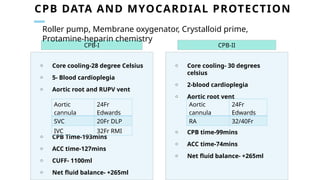
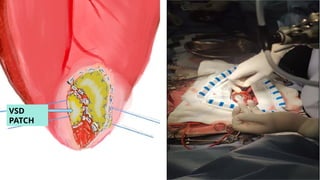
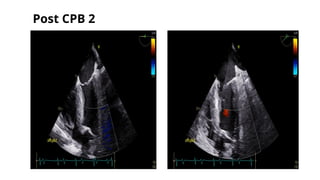
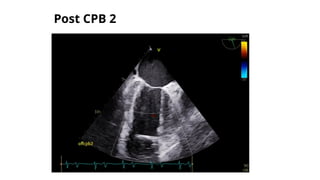
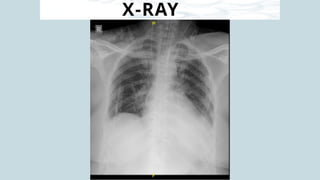
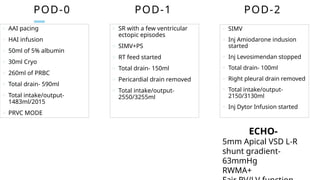
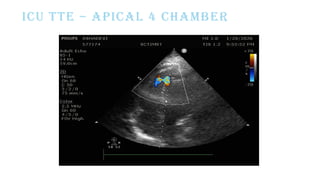
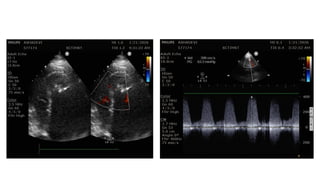
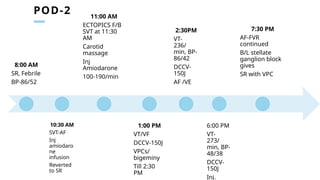
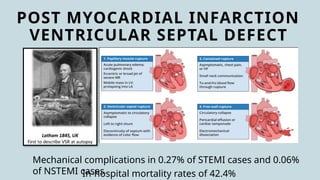
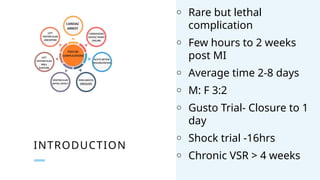
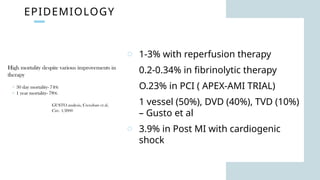
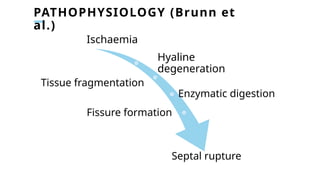
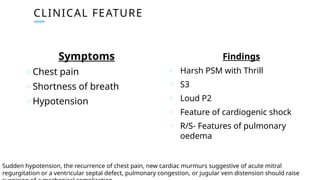
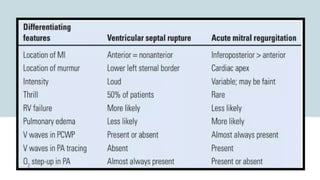
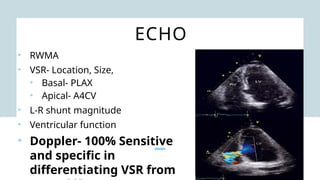
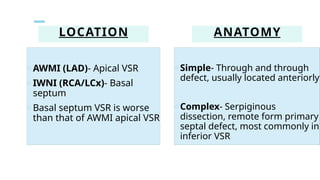
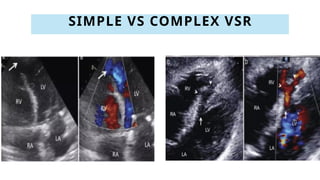
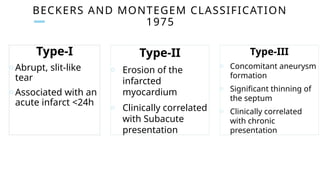
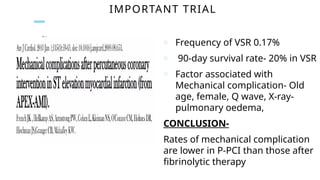
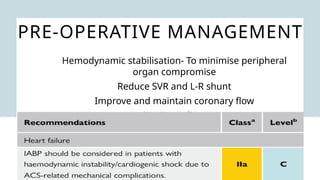
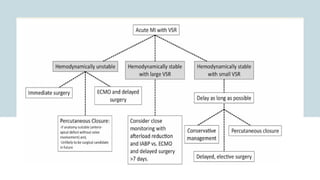
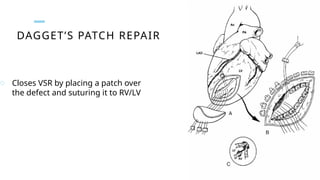
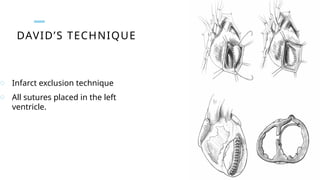
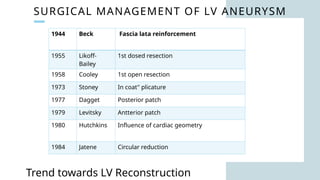
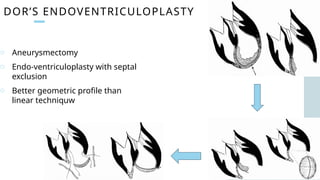
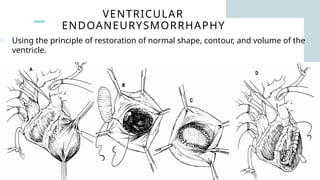
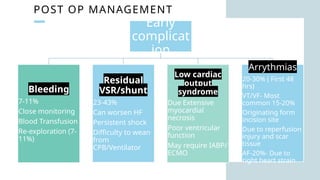
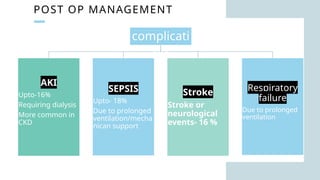
Ventricular septal rupture is one of the most lethal complication of myocardial infoarction with mortality rate upto 42.4% even after so much advancement in critical care and revascularisation therapy. The management of VSR is depends upon the hemodynamic stability of the patient.