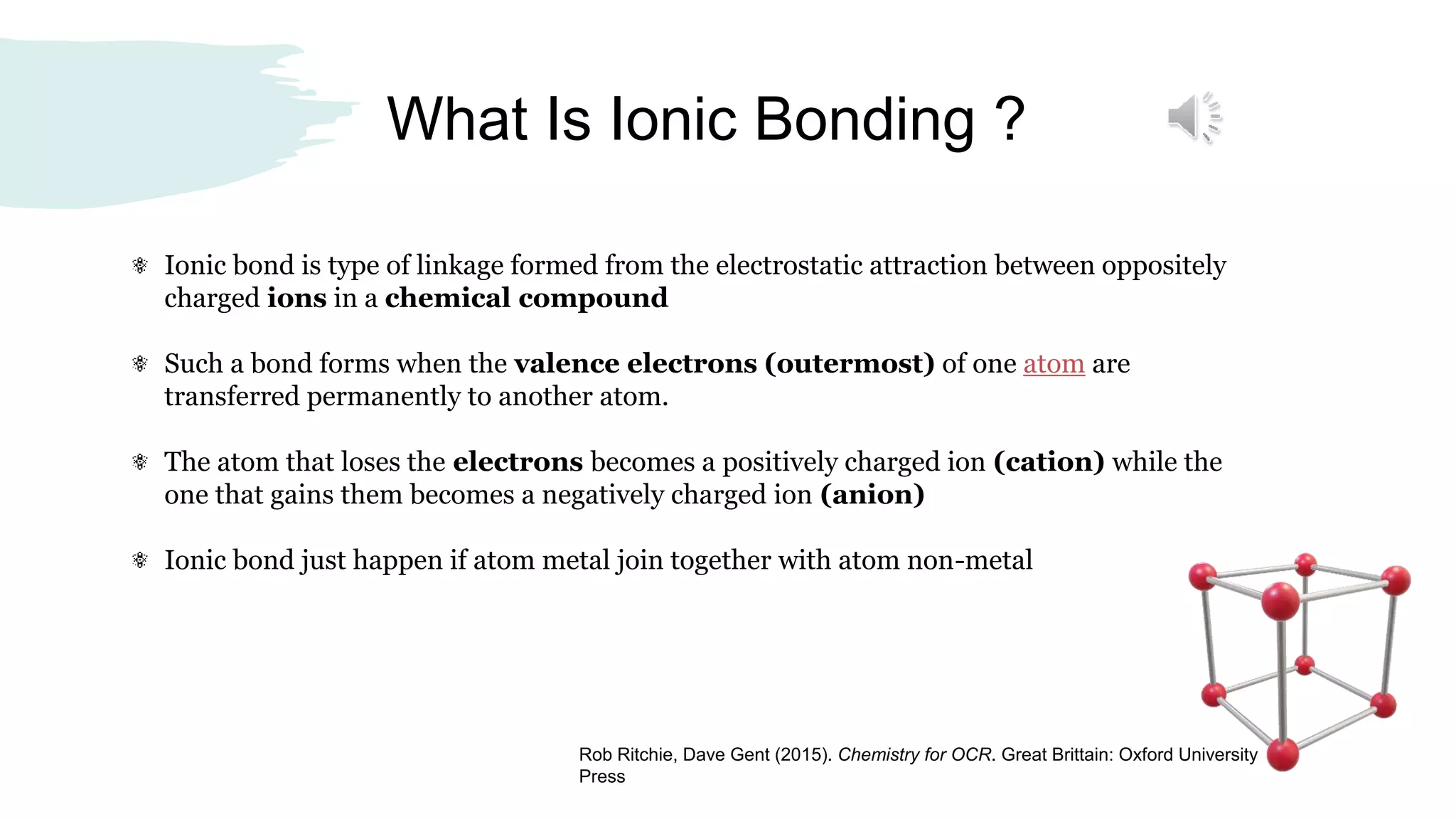
Ionic bonding occurs when valence electrons are transferred from metal atoms to non-metal atoms, resulting in positively charged cation and negatively charged anion ions that are attracted via electrostatic forces. Ionic compounds form when a metal donates its valence electrons to a nonmetal. The electrostatic attraction between opposite charges holds the ions together. Common ionic compounds include sodium chloride, formed from sodium cations and chloride anions, and magnesium oxide, formed from magnesium cations and oxide anions.





![Magnesium Oxide
Ryan, L., & Norris, R. (2014). Cambridge International AS and A Level Chemistry Coursebook.
Cambridge: Cambridge University Press.
When magnesium reacts with oxygen to form magnesium oxide, the two electrons in the outer shell of
each magnesium atom are transferred to the incompletely filled orbitals of an oxygen atom.
By losing two electrons, each magnesium atom achieves the electronic configuration [2,8]. By gaining
two electrons, each oxygen atom achieves the electronic configuration [2,8].
The ionic compound Sodium Chloride are held together by strong electrostatic forces between
oppositely charged ions.
These forces are referred to as ionic bonds
Magnesium Oxide has a higher melting point than sodium chloride because 2 electrons are
transferred therefore the forces of attraction are stronger.](https://image.slidesharecdn.com/ioniccompound-201002145610/75/Ionic-compound-7-2048.jpg)

