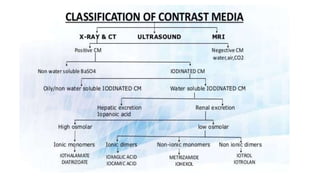
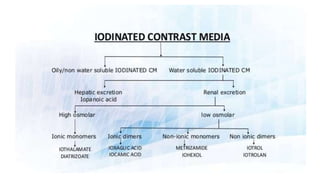
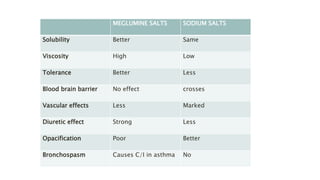
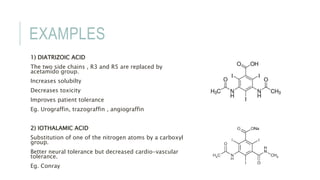
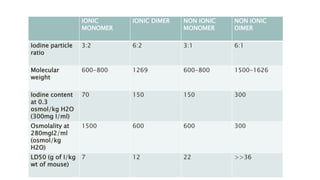
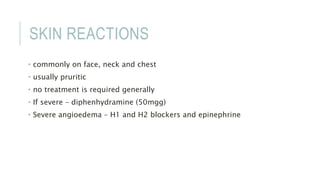
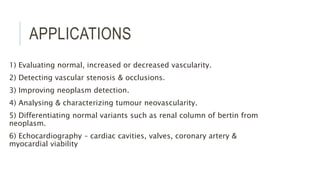
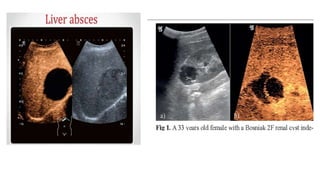
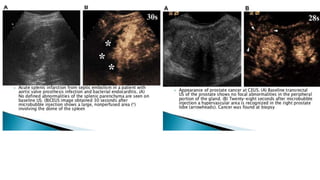
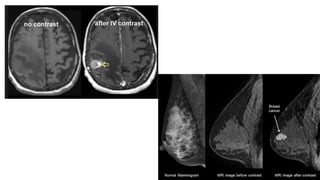
This document provides information on various intravenous contrast agents used in radiology. It discusses iodinated contrast agents used for CT imaging, classified based on their ionicity and molecular structure. Adverse reactions and safety considerations are covered. Contrast agents used for ultrasound imaging are also mentioned. The document concludes with details about MRI contrast agents, which alter proton relaxation to brighten or darken images. Paramagnetic and superparamagnetic agents commonly used are gadolinium and iron oxide particles respectively.