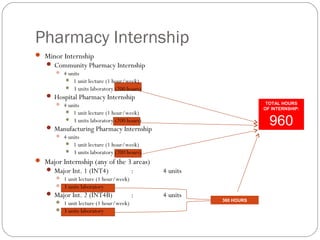
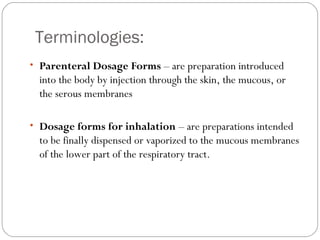
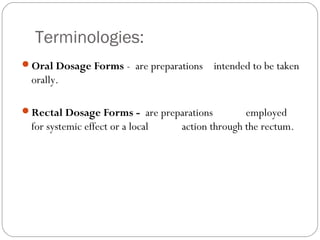
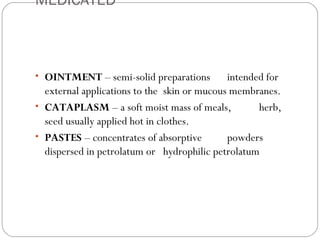
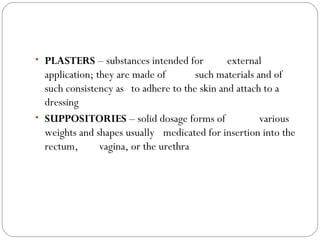
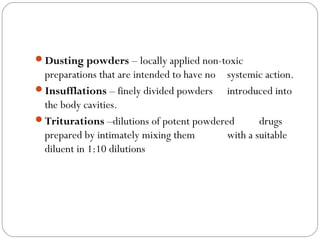
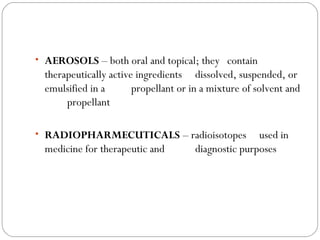
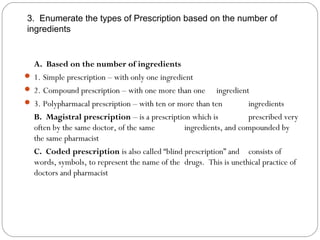
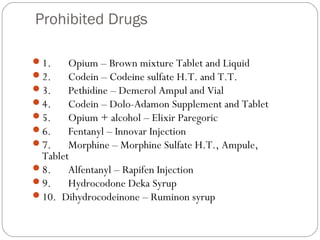
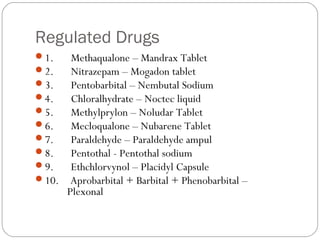
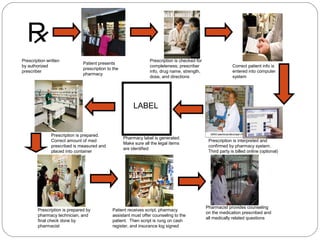
This document provides information about pharmacy internship programs and pharmaceutical dosage forms. It describes minor and major internships in community pharmacy, hospital pharmacy, and manufacturing pharmacy. It also outlines the specific objectives of community pharmacy internships. The document then discusses various pharmaceutical dosage forms including liquids, emulsions, suspensions, gels, and oral solid dosage forms like tablets and capsules. It provides examples of different dosage forms.