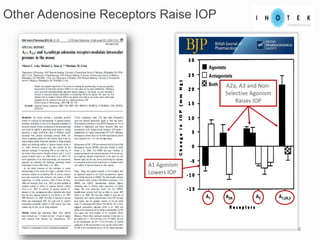
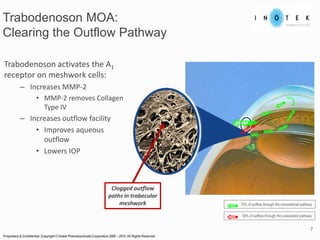
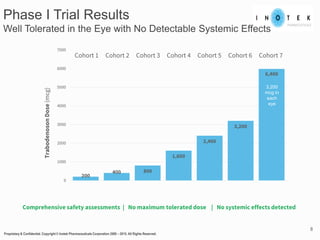
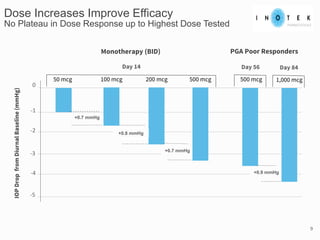
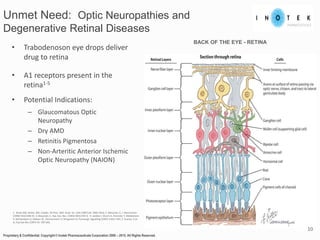
This presentation discusses forward-looking statements and risks associated with them. It notes that while management believes expectations in forward-looking statements are reasonable, actual results could differ materially due to known and unknown risks and uncertainties. The presentation also notes that views expressed as of the date may change in the future.