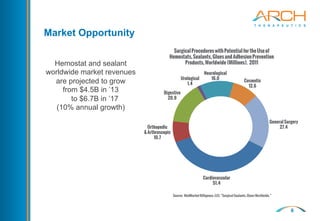
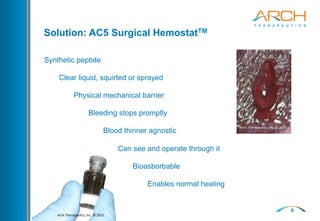
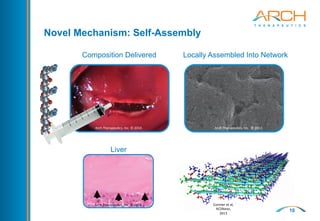
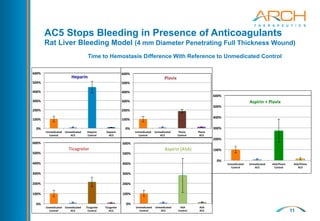
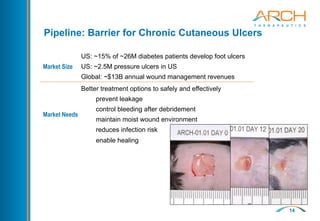
The document discusses a new hemostatic device called AC5 for faster and safer surgery. It notes the large market opportunity for hemostats and sealants which is projected to grow to $6.7 billion by 2017. The device uses a self-assembling peptide technology that creates a hemostatic barrier and has shown effectiveness in animal studies even in the presence of blood thinners.