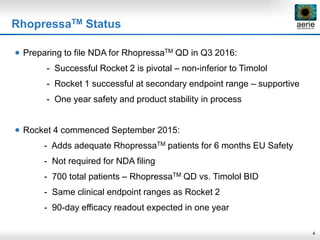
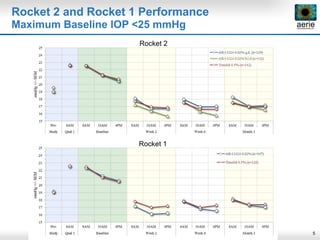
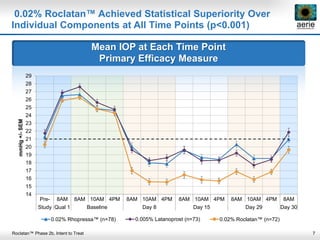
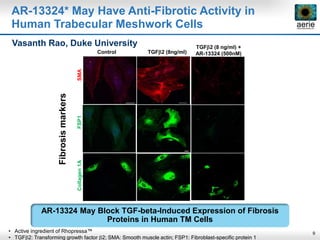
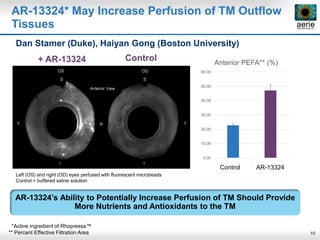
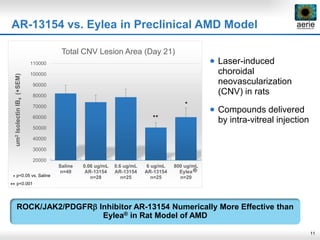
Aerie Pharmaceuticals is developing Rhopressa and Roclatan as once-daily eye drops to lower intraocular pressure for glaucoma. Rhopressa demonstrated efficacy in Phase 3 trials and Aerie expects to file an NDA in Q3 2016. Roclatan achieved all endpoints in a Phase 2b trial and is now in Phase 3 trials. Preclinical research also shows Rhopressa may modify diseased tissue and increase blood flow in the eye. Aerie is exploring AR-13154 for wet AMD which showed lesion size reduction exceeding the leading product in studies. The company collaborates with other organizations on delivery technologies and product candidates for the front and back of the eye.